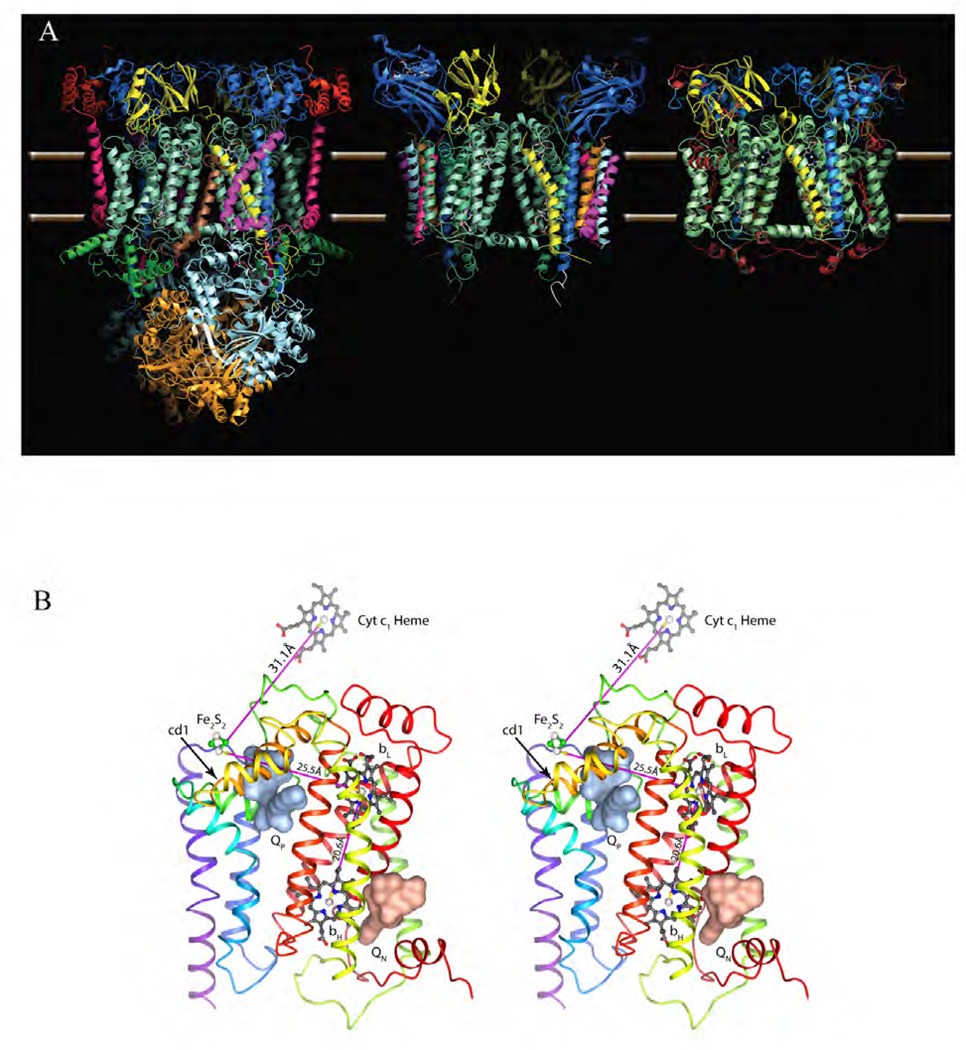
Figure 2. Atomic model of cyt bc1/b6f complexes.
(A) Comparison of the dimeric complexes with their respective 11, 8 and 3 subunits per monomer found in the crystal structures: bovine mitochondrial bc1, Chlamydomonas Reinhardtii algal b6f and Rhodobacter Sphaeroides bc1 with the three essential subunits: cyt b (green-cyan), cyt c1 (dark blue) and the iron-sulfur-protein (yellow). Subunit IV of R.S. bc1 was not found in the crystal structure. (B) Stereo diagram depicting cyt b with its eight TM helices and two b-type heme group bL and bH. The QP and QN site are occupied by stigmatellin (pale red surface) and antimycin (pale blue surface), respectively. Also shown are the iron-sulfur cluster Fe2S2 and the heme group of cyt c1. The magenta arrows indicate the shortest connection between the redox centers.