Abstract
A variety of studies have suggested that vitamin D may play a palliative role in improving insulin secretion and glucose tolerance. Endothelial cells of the microcirculation are thought to play an important role in regulating both insulin secretion and insulin sensitivity in target tissues. We have selectively deleted the vitamin D receptor (VDR) gene in endothelial cells of the murine vasculature. These mice demonstrate improved glucose tolerance, improved insulin sensitivity in skeletal muscle, but not in liver, and a reduction in expression and secretion of insulin in the pancreatic islets. Collectively, these data, taken within the context of recent publications in this field, suggest that the endothelial cell VDR plays a tonic inhibitory role in regulating glucose disposal and could prove to be a factor in controlling glucose homeostasis in the intact organism.
Keywords: Vitamin D Receptor, Endothelial Cell, Diabetes, Insulin Sensitivity
INTRODUCTION
Type 2 diabetes mellitus is a disorder that is usually associated with insulin resistance in target tissues like muscle and liver. Over time, this insulin resistance, coupled with eventual failure of beta cells to mount a compensatory insulin secretory response, leads to frank hyperglycemia. Though insulin resistance in conventional target tissues has been studied extensively [1], there is also evidence that insulin signaling may be impaired in cells that do not, themselves, participate to a significant degree in the metabolic disposal of glucose. Endothelial cells, for example, have been invoked as playing an important role in regulating insulin and glucose access to muscle beds, thereby indirectly controlling systemic insulin “sensitivity” [2].
Recent studies suggest that vitamin D may play an important role in the regulation of activity that extends well outside its accepted role in mineral homeostasis. Vitamin D deficiency has been linked to immunological [3] as well as cardiovascular dysfunction [4] and, importantly, to the development of both type 1 [5, 6] and type 2 diabetes [7]. Global deletion of the murine VDR gene has been shown to result in a negative effect [8] or no effect [9, 10] on glucose tolerance and incident diabetes. A number of cross sectional, human studies have demonstrated an inverse relationship between plasma levels of 25 hydroxyvitamin D and insulin resistance [11], metabolic syndrome [12, 13], as well as diabetes mellitus [14, 15]. A smaller number of longitudinal, observational studies have suggested a link between low vitamin D levels and incident diabetes, and others have suggested that intervention with vitamin D supplementation may serve to improve insulin resistance in selected patient populations [16], although this remains highly controversial [17].
We carried out a targeted deletion of the vitamin D receptor gene in the murine endothelial cell with a transgene expressing the Cre recombinase under the control of the Tie2 gene promoter (Tie-2Cre) to assess the role of this hormonal system in governing systemic insulin sensitivity. By way of contrast to previous studies in the whole animal VDR gene knockout [8] and VDR gene knockout in macrophage [33], VDR gene deletion in the endothelial cell (VDRL/L-Tie-2Cre+) led to improved glucose tolerance and insulin sensitivity in skeletal muscle but not liver.
MATERIALS AND METHODS
Endothelial Cell–Selective VDR Knockout Mice
Generation of endothelial cell–selective VDR gene knockout mice (VDRL/L-Tie-2Cre+ mice) in the C57Bl6 mouse strain was performed by crossing VDR exon 4 floxed mice (VDRL/L mice) with Tie-2Cre mice, as reported previously [18]. Of note, Tie-2Cre has also been shown to mediate targeted gene deletion in macrophages (see below for further discussion). Experiments were performed in mice at ~24 weeks of age. All animal-related experimental protocols were approved by the Institutional Animal Care and Use Committee at UCSF.
Blood Glucose Measurement
Blood glucose levels were measured in tail blood using the One Touch Ultra Blood Glucose Monitoring System (LifeScan, Inc.).
Glucose Tolerance Test (GTT) and Insulin Tolerance Test (ITT)
Glucose tolerance tests were performed in mice after an overnight fast. Blood glucose concentrations were measured before and 15, 30, 60, 120, and 240 minutes after an intraperitoneal glucose injection (2 g/kg). Insulin tolerance tests were carried out after an overnight fast, beginning between 9–10 AM. Recombinant human insulin was administered as an intraperitoneal bolus injection (0.75 U/kg; Eli Lilly and Company.). Blood glucose concentrations were measured before and 15, 30, 60, 90 and 180 minutes after injection.
In Vivo Insulin Sensitivity Test
Mice were fasted overnight before an intraperitoneal injection with human insulin (1 U/kg body weight) or saline. Eight minutes after injection, hindlimb skeletal muscle and liver were removed and snap frozen in liquid nitrogen in preparation for Western blotting.
Western Blotting
Mouse liver and skeletal muscle extracts were subjected to Western blot analysis as described previously [18]. P-AKT (phospho-Ser473, Cat# 4058) and total AKT (Cat# 9272) expression were detected using antibodies from Cell Signaling Technologies, Danvers, MA. Blots were then incubated with the appropriate horseradish peroxidase-linked secondary antibody, and ECL® reagent (Amersham Life Sciences, Arlington Heights, IL, U.S.A.) was used to visualize bands.
Islet Isolation and Ex Vivo Glucose-stimulated Insulin Secretion
Mouse islet isolation was performed as described previously [19] in the Islet Production Facility Core of the UCSF Diabetes Center. Equivalent sized islets were collected into modified Krebs-Ringer bicarbonate buffer (KRBB) containing 30 mg/dl glucose and cultured for 1 hour at 37°C. Glucose stimulated insulin secretion from isolated islets has been described previously [20]. Briefly, for each group, 10 islets were incubated for 30 minutes in 30 mg/dl or 300 mg/dl glucose in KRBB. Supernatants were collected to quantify secreted insulin using the Insulin EIA kit (ALPCO).
In Vivo Insulin Secretion
Serum insulin levels were measured at baseline and 30 min after glucose challenge (3 g/kg, i.p.). Blood was collected from the tail vein and centrifuged to generate serum. Insulin concentration was assessed using an Insulin EIA kit (ALPCO).
Quantitative PCR
RNA from isolated islets was obtained using QIAshredder (QIAGEN) and RNeasy Mini Kit (QIAGEN) according to manufacturer’s instructions. RNA concentration was measured by NanoDrop (Thermo Scientific).
Equal amounts of RNA were used to reverse transcribe cDNA with SuperScript® III Reverse Transcriptase (Life Technologies). Real-time quantitative PCR was performed using Applied Biosystems 7900 HT (Applied Biosystems, Foster City, CA). Expression levels of genes of interest were quantified and normalized to cyclophilin or GAPDH gene expression, as described previously [18]. Sense and anti-sense primers for SYBR®-Green based detection were as follows: mouse VDR (sense: AGGACAACCGGCGACACT and antisense: CTTACGCTGCACCTCCTCAT), insulin (sense: GAAGTGGAGGACCCACAAGTG and antisense: AAGGTCTGAAGGTCACCTGCTC) and glucagon (sense: GGCCAGGCAGCAAAGGA and antisense: TTCTTCTGGGAAGTCTCGCC).
Statistical Analysis
Data were analyzed by 2-way ANOVA or Student’s t-test. P values < 0.05 were considered significant.
RESULTS
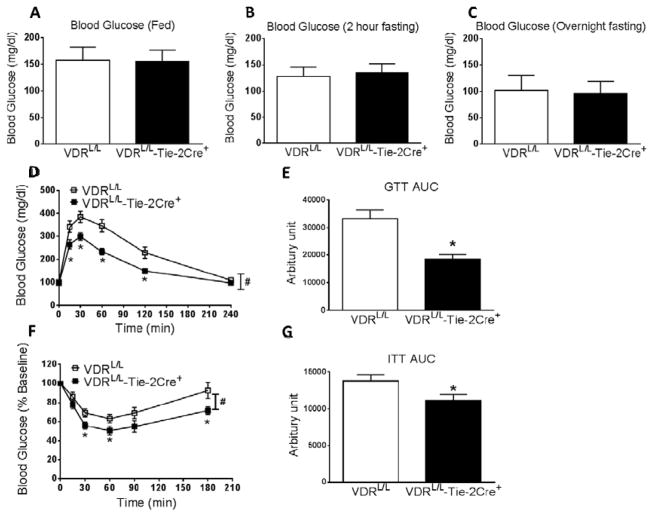
Basal blood glucose levels in fed or fasting (2 hrs or overnight) 24-week old mice were not different between the VDRL/L-Tie-2Cre+ mice and their littermate controls (VDRL/L) (Fig. 1A–C). However, when subjected to a glucose challenge, the VDRL/L-Tie-2Cre+ mice showed statistically significant lower blood glucose levels (ie, improved glucose tolerance) relative to the VDRL/L controls (Fig. 1D and E). When the same mice were subjected to an insulin tolerance test, blood glucose levels fell in both groups of mice but they fell to a greater degree in the VDRL/L-Tie-2Cre+ vs. VDRL/L mouse (Figs 1F and G), suggesting that the former have improved insulin sensitivity compared with the latter and offering a plausible explanation for the improved glucose tolerance.
Figure 1.
VDRL/L-Tie-2Cre+ mice have normal blood glucose levels, but increased glucose disposal and insulin sensitivity, at 24 weeks of age. A. Blood glucose in fed mice (n=7). B. Blood glucose after 2 hours of fasting (n=8). C. Blood glucose after overnight fasting (n=19). D. Glucose tolerance test (n=19). *p<0.05 vs. VDRL/L at indicated time point. # p=0.002 vs. VDRL/L assessed by two-way ANOVA for repeated measurements. E. The area under the curve (AUC) for glucose levels during glucose tolerance tests (n=19). *p<0.05 vs. VDRL/L. F. Insulin tolerance test (n=14–18). *p<0.05 vs. VDRL/L at indicated time point. # p=0.029 vs. VDRL/L assessed by two-way ANOVA for repeated measurements. G. The AUC of glucose levels during insulin tolerance tests (n=14–18). *p<0.05 vs. VDRL/L.
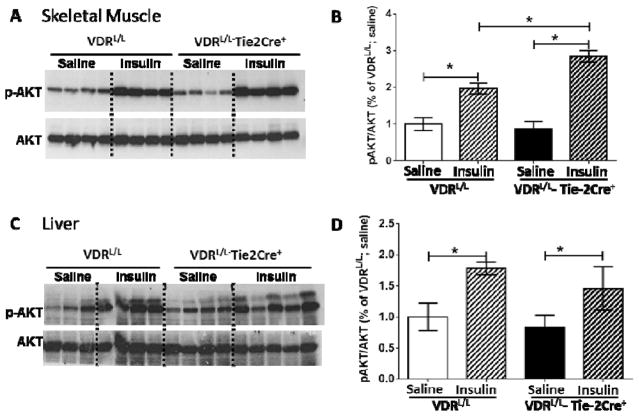
To explore the issue of differential insulin sensitivity further, we challenged both groups of mice with an insulin bolus, then collected tissue (liver and skeletal muscle), generated soluble tissue extracts and subjected these extracts to Western blot analysis to measure phospho-AKT (p-AKT) and total AKT levels. AKT is kinase positioned downstream in the insulin signal transduction pathway. Phosphorylation of AKT is a signature of insulin action. As shown in Fig. 2 basal levels of p-AKT, normalized for total AKT levels, were similar in liver and muscle extracts from VDRL/L-Tie-2Cre+ vs. VDRL/L mice. Following insulin administration, there was a significant increase in the p-AKT/total AKT ratio in the skeletal muscle extracts from VDRL/L mice, and this increase was amplified further in the VDRL/L-Tie-2Cre+ mice. Insulin administration also increased the p-AKT/total AKT ratio in liver extracts but, in this case, there was no difference in the ratio between VDRL/L and VDRL/L-Tie-2Cre+ mice. Collectively, these data suggest that the improved insulin sensitivity noted in Figs. 1F and G results, at least in part, from increased insulin signaling in skeletal muscle in the VDRL/L-Tie-2Cre+ mice.
Figure 2.
VDR deletion in endothelial cells increases insulin sensitivity in skeletal muscle but not in liver. Western blot analysis and quantification of p-AKT and total AKT in skeletal muscle (A) or liver (B) collected from VDRL/L and VDRL/L-Tie-2Cre+ mice after saline or insulin bolus injection (n=3–5). B. Western blot analysis and quantification of p-AKT and total AKT in liver collected from VDRL/L and VDRL/L-Tie-2Cre+ mice after insulin vs. saline injection (n=3–5). Data analyzed by 2-way ANOVA; *p<0.05.
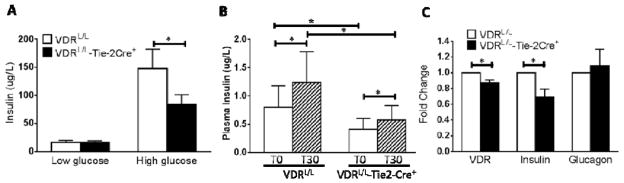
The observed improvement in glucose tolerance in the VDRL/L-Tie-2Cre+ mice could result from some combination of enhanced insulin secretion or improved insulin sensitivity in target tissues. The latter has been documented above. To address the former, we isolated islets from VDRL/L-Tie-2Cre+ vs. VDRL/L mice and examined insulin secretion under basal conditions and following exposure to high extracellular glucose. As shown in Fig. 3A basal insulin secretion from freshly isolated islets was equivalent in the two groups of mice and in both cases, glucose triggered a significant increase in insulin release; however, the increment seen in VDRL/L was significantly higher than that seen in VDRL/L-Tie2-Cre+ mice. We then looked at circulating serum insulin levels in vivo both at baseline and 30 min following glucose administration. As shown in Fig. 3B, plasma insulin levels in the VDRL/L mouse at baseline were roughly twice those found in the VDRL/L-Tie2-Cre+. Both mice demonstrated a significant increase in plasma insulin levels 30 min following glucose administration; however, the response in VDRL/L was more robust than that in the VDRL/L-Tie2-Cre+ and the ~2 fold difference in mean plasma insulin levels was maintained. Extrapolating this to the level of mRNA expression we saw a modest, but statistically significant decrease in VDR mRNA levels in the islets from VDRL/L-Tie2-Cre+ vs. VDRL/L mice, likely reflecting the fact that the endothelial cells represent a minor fraction of the total islet cell population. This was accompanied by a significant reduction in insulin mRNA levels without a significant change in expression of the preproglucagon gene (Fig. 3C).
Figure 3.
Diminished glucose-induced insulin secretion in islets from VDRL/L-Tie-2Cre+. A. Insulin secreted from 10 islets in 500 ul medium during 30 minute incubation with low glucose (30 mg/dl) or high glucose (300 mg/dl) (n=8). Open bar= VDRL/L, solid bar= VDRL/L-Tie-2Cre+. B. Plasma insulin levels before and 30 min after a bolus injection of glucose (3 g/kg) in VDRL/L vs. VDRL/L-Tie-2Cre+ mice (n=6). Open bar = time zero; hatched bar = 30 min. *p<0.05. C. Reduced VDR and insulin gene expression but unchanged pre-proglucagon gene expression in islets from VDRL/L-Tie-2Cre+ vs. VDRL/L mice (n=6–7). *p<0.05 vs. VDRL/L.
DISCUSSION
The present studies show that selective deletion of the VDR gene in murine endothelial cells results in an unpredicted improvement in glucose tolerance, without affecting basal glucose levels, and increased insulin sensitivity. This results in a secondary reduction in insulin secretion from beta cells in the pancreatic islets.
A broad based and growing literature suggests that vitamin D status, inferred from dietary intake of vitamin D or more directly from measurement of plasma 25 hydroxyvitamin D3 levels, is inversely related to the development of type 1 [13], as well as type 2[14, 15], diabetes mellitus. Vitamin D’s protective role in type 1 diabetes is assumed to be related to its immunosuppressive properties in reducing cytokine production, dendritic cell maturation and T cell function [3, 21]. Its role in type 2 diabetes is less well understood. There is evidence that the liganded VDR promotes beta cell secretory activity [22, 23] and may improve insulin sensitivity in peripheral target tissues [24], although this remains controversial [25, 26]. Both VDR and the ligand-generating 1-α-hydroxylase are found in beta cells of the islets [27, 28]. Vitamin D metabolites have been shown to suppress the islet renin-angiotensin system [29], a system which has been linked, secondarily, to beta cell dysfunction [30].
Since the Tie-2 Cre transgene has been previously shown to be expressed in the myeloid lineage [31] and the macrophage of this lineage is a well-known target of vitamin D action [32] it is possible that the effects on glucose control might be mediated, at least in part, by the deletion of the VDR in this cell type. It is noteworthy, however, that Oh et al [33] recently reported that deletion of VDR in macrophage results in increased insulin resistance – an effect which is opposite in direction to that identified here, lending additional support to our inference that it is the endothelial cell rather than the macrophage which is responsible for the observed phenotype.
The data presented here indicate that the improved glucose tolerance that is seen in VDRL/L-Tie-2Cre+ mice derives from increased insulin sensitivity in muscle but not liver. This may reflect differences in vascular morphology rather than fundamental differences in endothelial cell biology in these two tissues. Endothelial cells form tight junctions in capillaries perfusing skeletal muscle beds creating a functional barrier between the plasma column and the insulin-sensitive muscle cell. In liver, sinusoidal endothelial cells are typically separated by open fenestrations which limit their ability to establish a true blood-hepatocyte barrier. Therefore, based on vascular anatomy, one might predict that subtle changes in endothelial cell physiology or cytoarchitecture are more likely to exert important effects in relatively impermeant capillary networks like those in skeletal muscle vs. liver. Alternatively, the differences in insulin sensitivity could reflect differences in perfusion of the microcirculation in muscle. While blood pressure is unchanged in VDRL/L-Tie-2Cre+ mice at baseline [18], it remains possible that differential regulation of blood flow to muscle beds could result in increased glucose and insulin delivery and, inferentially, improvement in the observed “sensitivity” to insulin.
At one level the finding of improved glucose tolerance and insulin sensitivity in the VDRL/L-Tie-2Cre+ mouse seems counter-intuitive given the experimental and epidemiological data suggesting that the liganded receptor may be associated with improved insulin sensitivity [24, 34]. In the whole animal VDR gene knockout (VDR−/−), where effects on glucose tolerance have been observed, they have largely been negative in nature [8], implying that impaired insulin sensitivity in non-endothelial cells (eg, macrophages) [33] may dominate the phenotype. Nevertheless, these effects, even if idiosyncratic to the endothelial cell, will have to be accounted for in assessing response to vitamin D administration in the whole animal.
It is noteworthy that Kanda et al have seen a similar improvement in glucose tolerance in mice with endothelial cell selective deletion of the peroxisome proliferator activator receptor gamma (PPARγ) gene following administration of a high fat diet [35]. In this case they linked the improved insulin sensitivity in skeletal muscle to reduced CD36-dependent fatty acid transport across the endothelial barrier into the muscle interstitium. We have examined CD36 gene expression in our endothelial cell preparations but found no significant differences in cells from VDRL/L-Tie-2Cre+ vs. VDRL/L mice (data not shown).
Improved glucose tolerance could result from improved insulin sensitivity in the periphery and/or from improved insulin secretion from the beta cell. The results from the insulin tolerance test (Fig. 1) and the improved phospho-AKT response in skeletal muscle from the VDRL/L-Tie-2Cre+ mouse (Fig. 2) favor the former hypothesis. Our studies examining insulin secretion and insulin gene expression in pancreatic islets, as well as the reduction in circulating plasma insulin levels (Fig 3), support this hypothesis as well. Therefore, while there are clearly interactions between the beta cell and islet endothelial cells that foster normal islet development and insulin secretion [36], the changes observed here seem likely to reflect reduced insulin secretion due to improved insulin sensitivity in the periphery.
In summary, deletion of the VDR gene selectively in endothelial cells results in improved glucose tolerance, increased insulin sensitivity in skeletal muscle and reduced insulin production and secretion from pancreatic islets. Collectively, these studies add to the growing body of data suggesting that the VDR plays an important role in glucose homeostasis. Additional study will be required to assess the role that the liganded VDR plays in individual cell types to regulate glucose homeostasis in the whole animal.
Highlights.
We found improved glucose disposal in Tie-2Cre-mediated endothelial/macrophage VDR KO mice.
Improvement in glucose disposal is due to increased insulin sensitivity in skeletal muscle not in liver.
Reduced insulin secretion is likely secondary to improved insulin sensitivity.
Acknowledgments
Supported in part by NIH HL 45637 (DGG), HL-0861578 (DJG), a grant from the CeDAR Foundation and a grant from the UCSF Diabetes Center Family Fund.
Abbreviations
- VDR
vitamin D receptor
- VDRL/L mice
VDR gene exon 4-floxed mice
- VDRL/L-Tie-2Cre+ mice
VDR gene exon 4-floxed mice with Tie-2Cre Tg
- GTT
Glucose Tolerance Test
- ITT
Insulin Tolerance Test
- AUC
area under the curve
- KRBB
Krebs-Ringer bicarbonate buffer
Footnotes
Disclosures: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Muoio DM, Newgard CB. Molecular and metabolic mechanisms of insulin resistance and [beta]-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 2.Kubota T, Kubota N, Kumagai H, Yamaguchi S, Kozono H, Takahashi T, Inoue M, Itoh S, Takamoto I, Sasako T, Kumagai K, Kawai T, Hashimoto S, Kobayashi T, Sato M, Tokuyama K, Nishimura S, Tsunoda M, Ide T, Murakami K, Yamazaki T, Ezaki O, Kawamura K, Masuda H, Moroi M, Sugi K, Oike Y, Shimokawa H, Yanagihara N, Tsutsui M, Terauchi Y, Tobe K, Nagai R, Kamata K, Inoue K, Kodama T, Ueki K, Kadowaki T. Impaired Insulin Signaling in Endothelial Cells Reduces Insulin-Induced Glucose Uptake by Skeletal Muscle. Cell Metabolism. 2011;13:294–307. doi: 10.1016/j.cmet.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 3.Hewison M. An update on vitamin D and human immunity. Clinical Endocrinology. 2012;76:315–325. doi: 10.1111/j.1365-2265.2011.04261.x. [DOI] [PubMed] [Google Scholar]
- 4.Norman PE, Powell JT. Vitamin D and Cardiovascular Disease. Circ Research. 2014;114:379–393. doi: 10.1161/CIRCRESAHA.113.301241. [DOI] [PubMed] [Google Scholar]
- 5.Chakhtoura M, Azar ST. The Role of Vitamin D Deficiency in the Incidence, Progression, and Complications of Type 1 Diabetes Mellitus. International Journal of Endocrinology. 2013;2013:10. doi: 10.1155/2013/148673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong JY, Zhang W, Chen J, Zhang ZL, Han SF, Qin LQ. Vitamin D Intake and Risk of Type 1 Diabetes: A Meta-Analysis of Observational Studies. Nutrients. 2013;5:3551–3562. doi: 10.3390/nu5093551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forouhi NG, Ye Z, Rickard AP, Khaw KT, Luben R, Langenberg C, Wareham NJ. Circulating 25-hydroxyvitamin D concentration and the risk of type 2 diabetes: results from the European Prospective Investigation into Cancer (EPIC)-Norfolk cohort and updated meta-analysis of prospective studies. Diabetologia. 2012;55:2173–2182. doi: 10.1007/s00125-012-2544-y. [DOI] [PubMed] [Google Scholar]
- 8.Zeitz U, Weber K, Soegiarto DW, Wolf E, Balling R, Erben RG. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. The FASEB Journal. 2003 doi: 10.1096/fj.02-0424fje. [DOI] [PubMed] [Google Scholar]
- 9.Gysemans C, van Etten E, Overbergh L, Giulietti A, Eelen G, Waer M, Verstuyf A, Bouillon R, Mathieu C. Unaltered Diabetes Presentation in NOD Mice Lacking the Vitamin D Receptor. Diabetes. 2008;57:269–275. doi: 10.2337/db07-1095. [DOI] [PubMed] [Google Scholar]
- 10.Mathieu C, Van Etten E, Gysemans C, Decallonne B, Kato S, Laureys J, Depovere JOS, Valckx D, Verstuyf A, Bouillon R. In Vitro and In Vivo Analysis of the Immune System of Vitamin D Receptor Knockout Mice. Journal of Bone and Mineral Research. 2001;16:2057–2065. doi: 10.1359/jbmr.2001.16.11.2057. [DOI] [PubMed] [Google Scholar]
- 11.Esteghamati A, Aryan Z, Esteghamati AR, Nakhjavani M. Vitamin D Deficiency is Associated with Insulin Resistance in Nondiabetics and Reduced Insulin Production in Type 2 Diabetics. Horm Metab Res. 2012 doi: 10.1055/s-0034-1389903. [DOI] [PubMed] [Google Scholar]
- 12.JSJHKdH Blood Vitamin D Status and Metabolic Syndrome in the General Adult Population: A Dose-Response Meta-Analysis. The Journal of Clinical Endocrinology & Metabolism. 2014;99:1053–1063. doi: 10.1210/jc.2013-3577. [DOI] [PubMed] [Google Scholar]
- 13.Mitri J, Nelson J, Ruthazer R, Garganta C, Nathan DM, Hu FB, Dawson-Hughes B, Pittas AG. Plasma 25-hydroxyvitamin D and risk of metabolic syndrome: an ancillary analysis in the Diabetes Prevention Program. Eur J Clin Nutr. 2014;68:376–383. doi: 10.1038/ejcn.2013.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Husemoen LLN, Skaaby T, Thuesen BH, Jorgensen T, Fenger RV, Linneberg A. Serum 25(OH)D and incident type 2 diabetes: a cohort study. Eur J Clin Nutr. 2012;66:1309–1314. doi: 10.1038/ejcn.2012.134. [DOI] [PubMed] [Google Scholar]
- 15.Stadlmayr A, Aigner E, Huber-Schönauer U, Niederseer D, Zwerina J, Husar-Memmer E, Hohla F, Schett G, Patsch W, Datz C. Relations of vitamin D status, gender and type 2 diabetes in middle-aged Caucasians. Acta Diabetologica. 2014:1–8. doi: 10.1007/s00592-014-0596-9. [DOI] [PubMed] [Google Scholar]
- 16.Belenchia AM, Tosh AK, Hillman LS, Peterson CA. Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: a randomized controlled trial. The American Journal of Clinical Nutrition. 2013;97:774–781. doi: 10.3945/ajcn.112.050013. [DOI] [PubMed] [Google Scholar]
- 17.George PS, Pearson ER, Witham MD. Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta-analysis. Diabetic Medicine. 2012;29:e142–e150. doi: 10.1111/j.1464-5491.2012.03672.x. [DOI] [PubMed] [Google Scholar]
- 18.Ni W, Watts SW, Ng M, Chen S, Glenn DJ, Gardner DG. Elimination of Vitamin D Receptor in Vascular Endothelial Cells Alters Vascular Function. Hypertension. 2014;64:1290–1298. doi: 10.1161/HYPERTENSIONAHA.114.03971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szot GL, Koudria P, Bluestone JA. Murine Pancreatic Islet Isolation. 2007:e255. doi: 10.3791/255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Regard JB, Kataoka H, Cano DA, Camerer E, Yin L, Zheng YW, Scanlan TS, Hebrok M, Coughlin SR. Probing cell type–specific functions of Gi in vivo identifies GPCR regulators of insulin secretion. The Journal of Clinical Investigation. 2007;117:4034–4043. doi: 10.1172/JCI32994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolden-Kirk H, Overbergh L, Christesen HT, Brusgaard K, Mathieu C. Vitamin D and diabetes: Its importance for beta cell and immune function. Molecular and Cellular Endocrinology. 2011;347:106–120. doi: 10.1016/j.mce.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Kadowaki S, Norman AW. Dietary vitamin D is essential for normal insulin secretion from the perfused rat pancreas. The Journal of Clinical Investigation. 1984;73:759–766. doi: 10.1172/JCI111269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luca G, Calvitti M, Neri LM, Becchetti E, Capitani S, Basta G, Angeletti G, Fanelli C, Brunetti P, Calafiore R. Sertoli cell-induced reversal of adult rat pancreatic islet beta-cells into fetal-like status: potential implications for islet transplantation in type I diabetes mellitus. J Investig Med. 2000;48:441–448. [PubMed] [Google Scholar]
- 24.von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient – a randomised, placebo-controlled trial. British Journal of Nutrition. 2010;103:549–555. doi: 10.1017/S0007114509992017. [DOI] [PubMed] [Google Scholar]
- 25.Liu XJ, Wang BW, Zhang C, Xia MZ, Chen YH, Hu CQ, Wang H, Chen X, Xu DX. Vitamin D Deficiency Attenuates High-Fat Diet-Induced Hyperinsulinemia and Hepatic Lipid Accumulation in Male Mice. Endocrinology. 2015;156:2103–2113. doi: 10.1210/en.2014-2037. [DOI] [PubMed] [Google Scholar]
- 26.Kang S, Tsai LT, Zhou Y, Evertts A, Xu S, Griffin MJ, Issner R, Whitton HJ, Garcia BA, Epstein CB, Mikkelsen TS, Rosen ED. Identification of nuclear hormone receptor pathways causing insulin resistance by transcriptional and epigenomic analysis. Nat Cell Biol. 2015;17:44–56. doi: 10.1038/ncb3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bland R, Markovic D, Hills CE, Hughes SV, Chan SLF, Squires PE, Hewison M. Expression of 25-hydroxyvitamin D3-1α-hydroxylase in pancreatic islets. The Journal of Steroid Biochemistry and Molecular Biology. 2004;89–90:121–125. doi: 10.1016/j.jsbmb.2004.03.115. [DOI] [PubMed] [Google Scholar]
- 28.Johnson JA, Grande JP, Roche PC, Kumar R. Immunohistochemical localization of the 1,25(OH)2D3 receptor and calbindin D28k in human and rat pancreas. 1994 doi: 10.1152/ajpendo.1994.267.3.E356. [DOI] [PubMed] [Google Scholar]
- 29.Cheng Q, Li YC, Boucher BJ, Leung PS. A novel role for vitamin D: modulation of expression and function of the local renin–angiotensin system in mouse pancreatic islets. Diabetologia. 2011;54:2077–2081. doi: 10.1007/s00125-011-2100-1. [DOI] [PubMed] [Google Scholar]
- 30.Leung PS. The physiology of a local renin–angiotensin system in the pancreas. The Journal of Physiology. 2007;580:31–37. doi: 10.1113/jphysiol.2006.126193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Constien R, Forde A, Liliensiek B, Gröne HJ, Nawroth P, Hämmerling G, Arnold B. Characterization of a novel EGFP reporter mouse to monitor Cre recombination as demonstrated by a Tie2 Cre mouse line. genesis. 2001;30:36–44. doi: 10.1002/gene.1030. [DOI] [PubMed] [Google Scholar]
- 32.Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-Dihydroxyvitamin D3 Receptor in the Immune System. Archives of Biochemistry and Biophysics. 2000;374:334–338. doi: 10.1006/abbi.1999.1605. [DOI] [PubMed] [Google Scholar]
- 33.Oh J, Riek Amy E, Darwech I, Funai K, Shao J, Chin K, Sierra Oscar L, Carmeliet G, Ostlund Richard E, Jr, Bernal-Mizrachi C. Deletion of Macrophage Vitamin D Receptor Promotes Insulin Resistance and Monocyte Cholesterol Transport to Accelerate Atherosclerosis in Mice. Cell Reports. 2015;10:1872–1886. doi: 10.1016/j.celrep.2015.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The Effects of Calcium and Vitamin D Supplementation on Blood Glucose and Markers of Inflammation in Nondiabetic Adults. Diabetes Care. 2007;30:980–986. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 35.Kanda T, Brown JD, Orasanu G, Vogel S, Gonzalez FJ, Sartoretto J, Michel T, Plutzky J. PPARγ in the endothelium regulates metabolic responses to high-fat diet in mice. The Journal of Clinical Investigation. 2009;119:110–124. doi: 10.1172/JCI36233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eberhard D, Kragl M, Lammert E. ‘Giving and taking’: endothelial and β-cells in the islets of Langerhans. Trends in Endocrinology & Metabolism. 2010;21:457–463. doi: 10.1016/j.tem.2010.03.003. [DOI] [PubMed] [Google Scholar]