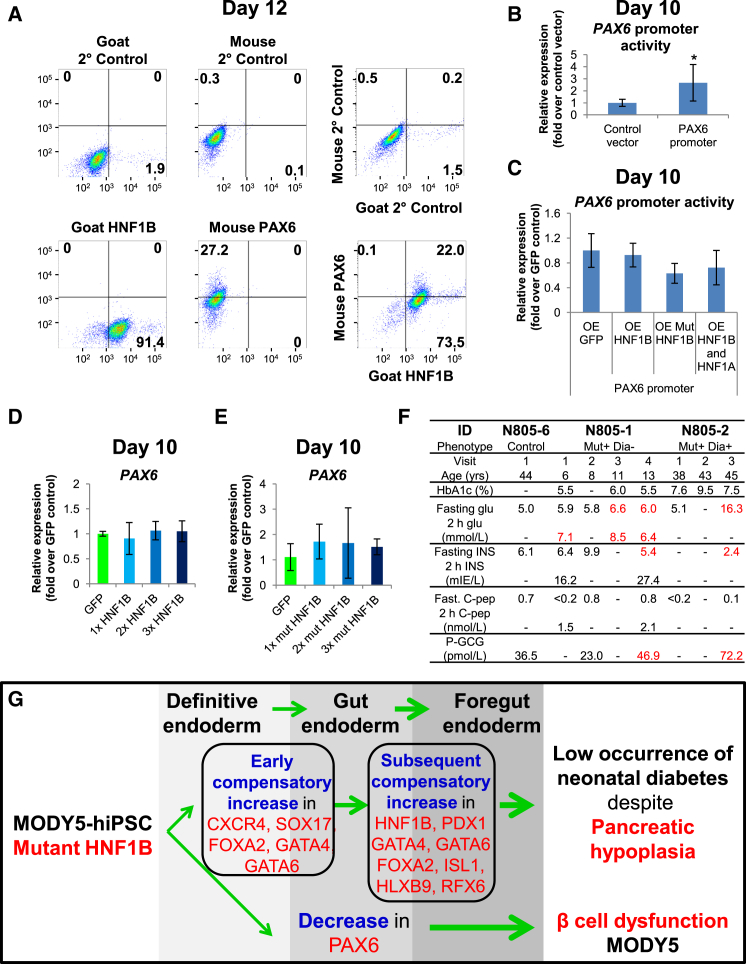
Figure 5.
HNF1B Is Not Directly Involved in Gene Regulation of PAX6
(A) The percentage of HNF1B+ and PAX6+ cells after differentiation of hiPSCs for 12 days.
(B and C) Luciferase assay showing (B) basal PAX6 promoter activity and (C) the effect of HNF1B and/or HNF1A overexpression on PAX6 promoter activity in hiPSCs differentiated for 10 days.
(D and E) Expression of PAX6 in hiPSCs differentiated for 10 days and overexpressing increasing amounts of (D) HNF1B or (E) mutant HNF1BS148L from days 7–10. The experiments in (B) to (E) were replicated at least twice. All error bars indicate SD of three biological replicates in an independent experiment. ∗p < 0.05 by two-sided Student's t test on three independent experiments.
(F) Clinical characteristics of MODY5 patients including HbA1c (%), blood glucose (glu), insulin (INS), C-peptide (C-pep), and plasma glucagon (P-GCG) concentrations.
(G) Model depicting the impact of mutant HNF1BS148L/+ on early human pancreas development. Mutant HNF1BS148L/+ evokes an early increase in definitive endoderm genes followed by a subsequent increase in pancreas-related foregut endoderm genes. Thus, although MODY5 patients typically develop pancreatic hypoplasia there is a low occurrence of neonatal diabetes. The decrease in early PAX6 gene expression in pancreatic progenitors may partly account for the subsequent β-cell dysfunction in MODY5 patients.