Summary
Hematopoietic stem cells (HSCs) originate from hemogenic endothelium within the aorta-gonad-mesonephros (AGM) region of the mammalian embryo. The relationship between genetic circuits controlling stem cell genesis and multi-potency is not understood. A Gata2 cis element (+9.5) enhances Gata2 expression in the AGM and induces the endothelial to HSC transition. We demonstrated that GATA-2 rescued hematopoiesis in +9.5−/− AGMs. As G-protein-coupled receptors (GPCRs) are the most common targets for FDA-approved drugs, we analyzed the GPCR gene ensemble to identify GATA-2-regulated GPCRs. Of the 20 GATA-2-activated GPCR genes, four were GATA-1-activated, and only Gpr65 expression resembled Gata2. Contrasting with the paradigm in which GATA-2-activated genes promote hematopoietic stem and progenitor cell genesis/function, our mouse and zebrafish studies indicated that GPR65 suppressed hematopoiesis. GPR65 established repressive chromatin at the +9.5 site, restricted occupancy by the activator Scl/TAL1, and repressed Gata2 transcription. Thus, a Gata2 cis element creates a GATA-2-GPCR circuit that limits positive regulators that promote hematopoiesis.
Graphical Abstract
Highlights
-
•
GATA-2 rescues +9.5−/− AGM hematopoietic activity
-
•
GATA-2 upregulates Gpr65, which encodes a negative regulator of hematopoiesis
-
•
GPR65 suppresses hematopoiesis by repressing Gata2 expression
-
•
GPR65 represses Gata2 expression by increasing H4K20me1, restricting Scl/TAL1 occupancy
In this report, Bresnick and colleagues describe the discovery of a circuit involving the actions of a transcription factor and a G-protein-coupled receptor to restrict hematopoiesis in the mouse embryo. The underlying mechanism involved establishment of a repressive chromatin mark at a vital cis-regulatory element of the Gata2 locus, which limits Scl/TAL1 occupancy at this element.
Introduction
Establishment and maintenance of the adult hematopoietic system requires the generation of hematopoietic stem cells (HSCs) from a unique endothelial cell (hemogenic) in the aorta-gonad-mesonephros (AGM) region of the mammalian embryo (Dzierzak and Speck, 2008). HSCs develop in clusters that bud off from hemogenic endothelium (Bertrand et al., 2010, Boisset et al., 2010), a process termed endothelial to hematopoietic transition (EHT). HSCs migrate to and colonize the fetal liver and, subsequently, the bone marrow (Orkin and Zon, 2008). Whereas major efforts have focused on defining regulatory proteins/networks governing EHT, many questions remain unanswered regarding the molecular constituents and mechanisms.
Master regulatory transcription factors co-localize at cis elements of target genes in hematopoietic stem and progenitor cells (HSPCs) to establish genetic networks that control hematopoiesis (Beck et al., 2013, Fujiwara et al., 2009, May et al., 2013, Tripic et al., 2008, Wilson et al., 2010, Wozniak et al., 2008, Yu et al., 2009). The combinatorial mechanisms operating in hemogenic endothelium, and the relationship between mechanisms governing EHT and HSC multi-potency, are unclear. A shared component of the mechanisms involves the transcription factor GATA-2, which is required for definitive hematopoiesis (Tsai et al., 1994). GATA-2 functions in hemogenic endothelium to induce EHT and regulates HSC function (de Pater et al., 2013, Gao et al., 2013, Johnson et al., 2012, Ling et al., 2004, Rodrigues et al., 2005).
Since GATA-2 induces EHT, it is instructive to consider factors/signals upstream of GATA-2. Deletion of a cis element 9.5 kb downstream of the Gata2 promoter (+9.5) in mice decreased Gata2 expression in AGM hemogenic endothelium, deregulated genes encoding positive regulators of hematopoiesis, and abrogated EHT (Gao et al., 2013). Deletion of a cis element 77 kb upstream of the promoter (−77) reduced Gata2 expression in myelo-erythroid progenitors and impaired progenitor function without affecting EHT (Johnson et al., 2015). The defective HSC generator of +9.5−/− embryos depleted HSPCs in the fetal liver and caused lethality at embryonic day 13–14 (E13–14) (Johnson et al., 2012). Since the +9.5 controls Gata2 expression and EHT (Gao et al., 2013, Hsu et al., 2013), and GATA-2 occupies the +9.5 (Fujiwara et al., 2009, Grass et al., 2006), one aspect of the +9.5 mechanism involves GATA-2-mediated positive autoregulation. Factors implicated upstream of GATA-2 include bone morphogenetic protein 4 (Lugus et al., 2007, Maeno et al., 1996), Notch signaling (Guiu et al., 2013, Robert-Moreno et al., 2008), the Ets factor Etv2 (Liu et al., 2015), and the methylcytosine dioxygenases Tet2/Tet3 (Li et al., 2015).
The Gata2 +9.5 site regulates a large gene cohort in hemogenic endothelium, and these genes do not parse into a single pathway (Gao et al., 2013). The genes include Runx1, Lyl1, and Mpl, positive mediators of HSC generation and/or function. The identification of vital constituents of the +9.5-dependent genetic network will reveal how a cis element triggers EHT. As GATA-2 lacks features that can be leveraged for drug binding, identifying +9.5 network components will reveal strategies to promote HSC generation/function for transplantation and inhibit leukemia cell proliferation and survival.
The most common targets for drugs approved by the Food and Drug Administration are GPCRs (Roth and Kroeze, 2015). The GPCR family consists of 341 non-olfactory receptors classified as rhodopsin, secretin, glutamate, adhesion, and frizzled/taste2, based on sequence homology (Lagerstrom and Schioth, 2008). CXCR4, a rhodopsin-like GPCR, recognizes SDF-1/CXCL-12 and controls HSPC survival, proliferation, migration, and engraftment (Broxmeyer et al., 2005). CXCR4 antagonists are used as mobilizing agents for stem cell transplantation. The prostaglandin PGE2, which functions through a group of related GPCRs (Sugimoto and Narumiya, 2007), expands HSPCs (Goessling et al., 2011, Hoggatt et al., 2013, North et al., 2007). We described +9.5-mediated upregulation of Gpr56 expression (Gao et al., 2013). Loss-of-function analyses indicated that GPR56 promotes EHT (Solaimani Kartalaei et al., 2015) and contributes to HSC maintenance (Saito et al., 2013). Galpha(s)-mediated signaling controls HSPC engraftment of bone marrow (Adams et al., 2009).
By profiling expression of the GPCR cohort in the AGM, we discovered a subset of GATA-2-regulated GPCRs and a GATA-2- and GATA-1-regulated cohort, including GPR65, which suppresses AGM hematopoiesis. The +9.5 site, and its regulated protein GATA-2, which positively regulate hematopoiesis, upregulated Gpr65 encoding a negative regulator of hematopoiesis. These results provide evidence for a GATA factor-GPCR type I incoherent feedforward loop as a vital component of the genetic network that controls HSPC generation and function.
Results
GATA-2 Expression in +9.5−/− AGM Rescues Hematopoiesis
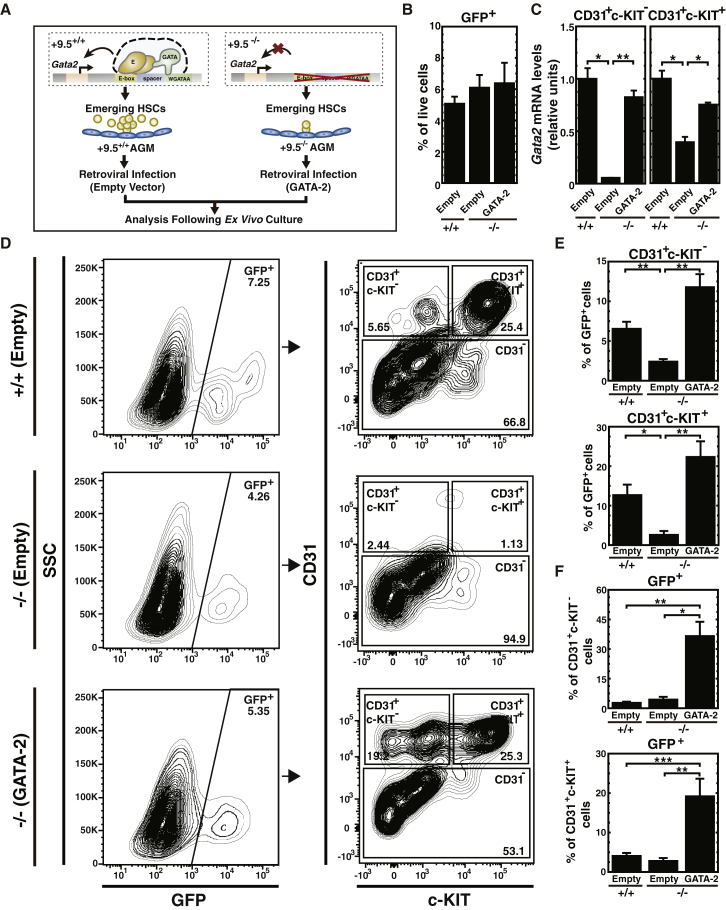
Deletion of the +9.5 site reduced Gata2 expression in AGM hemogenic endothelium and abrogated EHT (Gao et al., 2013) (Figure 1A). These results suggest that factors/signals conferring +9.5 activity control Gata2 expression, which promotes EHT. In principle, the +9.5 might regulate other genes in cis or in trans that control EHT. To determine whether the HSC generation defect of +9.5−/− AGM results from insufficient GATA-2 production, we tested whether GATA-2 expression rescues the defect. E11.5 mouse embryo AGMs were infected with GATA-2-expressing retrovirus (Figure 1A). After 96 hr of explant culture, we quantitated endothelial and hematopoietic cell populations in infected (GFP+) cells by flow cytometry using CD31 and c-KIT surface markers. The infection efficiency was similar among the three conditions (+9.5+/+-empty vector, +9.5−/−-empty vector, +9.5−/−-GATA-2), as indicated by the indistinguishable percentage of GFP+ live cells (Figure 1B). GATA-2 expression restored Gata2 mRNA to the wild-type level in CD31+c-KIT− endothelial and CD31+c-KIT+ hematopoietic cells (Figure 1C), and rescued both populations (Figures 1D and 1E). Since the infected cells express GFP, the percentage of GFP+ cells in each population increased in +9.5−/− AGM infected with GATA-2-expressing retrovirus (Figure 1F). However, retroviral-mediated GATA-2 expression did not rescue CD31+c-KIT− endothelial and CD31+c-KIT+ hematopoietic cell populations in the GFP− cells (Figure S1). Thus, the +9.5−/− AGM hematopoiesis defect resulted from insufficient GATA-2 production, is associated with reduced CD31+c-KIT− endothelial cells, and can be rectified by restoring GATA-2.
Figure 1.
GATA-2 Expression in +9.5−/− AGM Rescues CD31+c-KIT+ Hematopoietic and CD31+c-KIT− Endothelial Cells
(A) AGM ex vivo retroviral infection and culture.
(B) Flow cytometric analysis of GFP+ cells within total live cells (6 litters: +9.5+/+-Empty [n = 8 embryos]; +9.5−/−-Empty [n = 4 embryos]; +9.5−/−-GATA-2 [n = 6 embryos]).
(C) RT-PCR analysis of Gata2 mRNA levels in FACS-sorted CD31+c-KIT− and CD31+c-KIT+ cells (six litters: +9.5+/+-Empty [n = 8 embryos]; +9.5−/−-Empty [n = 4 embryos]; +9.5−/−-GATA-2 [n = 6 embryos]).
(D) Representative flow cytometric plots of CD31+c-KIT+ and CD31+c-KIT− cell populations in infected AGMs after 96 hr of ex vivo culture.
(E and F) Quantitation of flow cytometry data expressed as percentage of CD31+c-KIT− and CD31+c-KIT+ cells in GFP+ cells (E) and the percentage of GFP+ cells in CD31+c-KIT− and CD31+c-KIT+ cells (F) (6 litters: +9.5+/+-Empty [n = 8 embryos]; +9.5−/−-Empty [n = 4 embryos]; +9.5−/−-GATA-2 [n = 6 embryos]).
Error bars represent SEM. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 (two-tailed unpaired Student's t test).
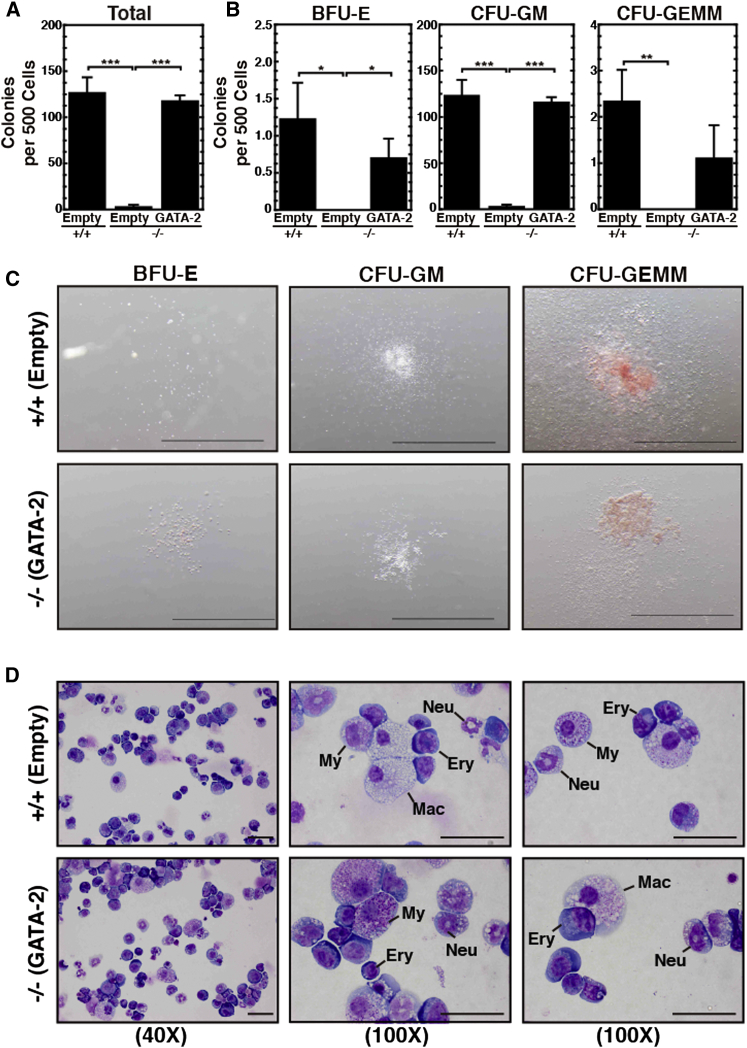
To assess whether the GATA-2-rescued CD31+c-KIT+ hematopoietic cells are functional, we measured their capacity to generate myelo-erythroid colonies in a colony-forming unit (CFU) assay. After culturing retroviral-infected AGMs for 96 hr, GFP+ CD31+c-KIT+ hematopoietic cells were isolated by fluorescence-activated cell sorting (FACS) and assayed for their capacity to generate BFU-E (erythroid burst-forming units), CFU-GM (granulocyte, macrophage), and CFU-GEMM (granulocyte, erythrocyte, monocyte/macrophage, megakaryocyte) colonies. Whereas +9.5−/− AGM failed to generate CFUs, GATA-2 expression in the +9.5−/− AGM induced CFUs comparable with +9.5+/+ AGM (Figure 2A). Quantification of colony types revealed comparable numbers of rescued CFU-GM colonies in comparison with wild-type AGM (Figure 2B). GATA-2 expression induced BFU-E and CFU-GEMM colonies (Figure 2B). Colonies derived from wild-type and rescued samples were morphologically indistinguishable (Figure 2C). Wright-Giemsa staining of cells from colonies revealed normal myeloid and erythroid cell generation from the GATA-2-expressing +9.5−/− AGM (Figure 2D). The GATA-2-induced CD31+c-KIT+ hematopoietic cells exhibited qualitatively and quantitatively normal activity.
Figure 2.
Rescued CD31+c-KIT+ Cells Exhibit Normal Colony-Forming Unit Activity
(A and B) Quantitative analysis of colony-forming activity of FACS-sorted CD31+c-KIT+ cells (6 litters: +9.5+/+-Empty [n = 9 embryos]; +9.5−/−-Empty [n = 7 embryos]; +9.5−/−-GATA-2 [n = 10 embryos]).
(C) Representative BFU-E, CFU-GM, and CFU-GEMM colonies from FACS-sorted CD31+c-KIT+ cells. Scale bar, 2 mm.
(D) Representative images of Wright-Giemsa-stained cells from colonies. Ery, erythroblast; Mac, macrophage; My, myeloid precursor; Neu, neutrophil. Scale bar, 40 μm.
Error bars represent SEM. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 (two-tailed unpaired Student's t test).
Global GPCR Analysis in the AGM: Discovery of a GATA Factor-Regulated GPCR Cohort
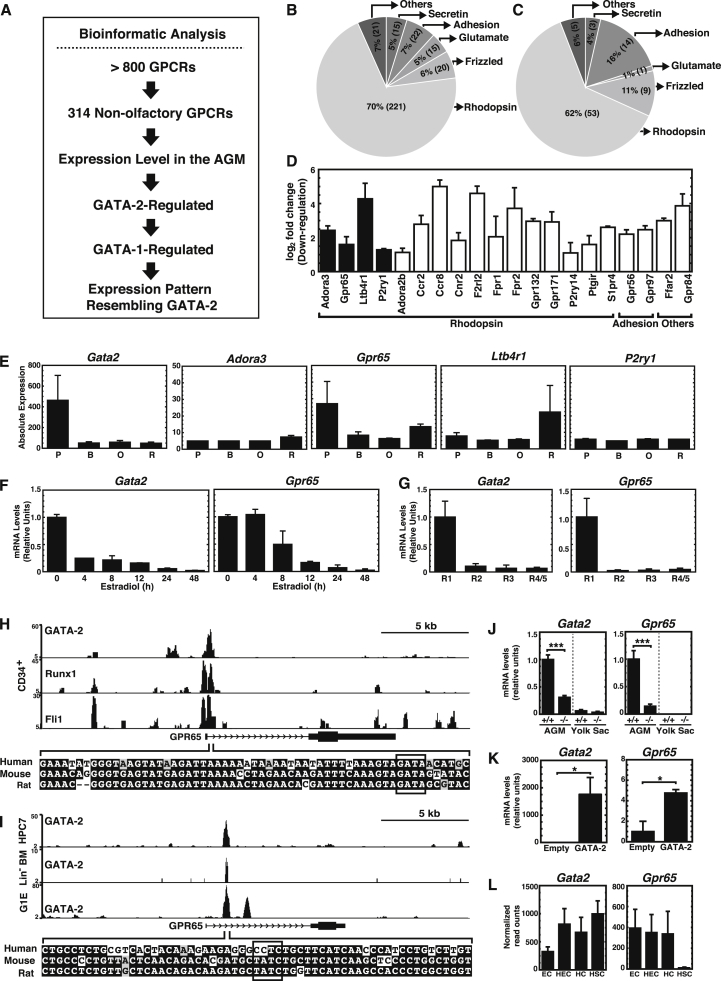
The +9.5 site confers Gata2 expression and establishes a genetic network involving known HSC regulators and genes not implicated in hematopoiesis (Gao et al., 2013). To discover vital constituents of the network, especially those with potential for modulation by small molecules/drugs, we systematically analyzed the expression pattern of non-olfactory GPCRs (Figure 3A). The human genome encodes greater than 800 GPCRs, which are categorized into rhodopsin, secretin, glutamate, adhesion and frizzled/taste2 families based on sequence homology; 341 are distinct from the olfactory and taste GPCRs (Lagerstrom and Schioth, 2008, Roth and Kroeze, 2015). Using our AGM RNA-sequencing (RNA-seq) data (Gao et al., 2013), we parsed AGM-expressed GPCRs into the canonical categories: secretin, 5% (15); adhesion, 7% (22); glutamate, 5% (15); frizzled/taste2, 6% (20); and rhodopsin, 70% (221) (Figure 3B).
Figure 3.
Global GPCR Analysis in the AGM
(A) Global GPCR analysis.
(B) Non-olfactory GPCRs (N = 314) were categorized into Secretin, Adhesion, Glutamate, Frizzled/Taste2, and Rhodopsin families based on sequence homology.
(C) Classification of 85 GPCRs expressed in the AGM (>5 transcripts per million) into five families.
(D) Bar graph depicting GATA-2-regulated genes from RNA-seq analysis of +9.5+/+ and +9.5−/− AGMs (Gao et al., 2013). Black bars, genes co-regulated by GATA-1 according to our prior microarray analysis of G1E-ER-GATA with or without β-estradiol treatment (DeVilbiss et al., 2013).
(E) Gata2, Adora3, Gpr65, Ltb4r1, and P2ry1 expression during erythropoiesis. B, basophilic erythroblast; O, polyorthochromatic erythroblast; P, proerythroblast; R, reticulocyte (http://www.cbil.upenn.edu/ErythronDB/).
(F) Time course of Gata2 and Gpr65 expression following β-estradiol treatment in G1E-ER-GATA cells (n = 3 independent experiments).
(G) RT-PCR analysis of Gata2 and Gpr65 in FACS-sorted R1, R2, R3, and R4/5 populations from fetal liver (n = 3 independent experiments).
(H and I) ChIP signal map for Gpr65 in human CD34 cells (H) (Beck et al., 2013), mouse HPC7 cells (Wilson et al., 2010), Lin− bone marrow cells (Li et al., 2011), and G1E cells (Trompouki et al., 2011) (I).
(J and K) RT-PCR analysis of Gata2 and Gpr65 mRNA in +9.5+/+ and +9.5−/− AGM (5 litters: +9.5+/+ [n = 8 embryos]; +9.5−/− [n = 6 embryos]) and yolk sac (three litters: +9.5+/+ [n = 7 embryos]; +9.5−/− [n = 5 embryos]) (J), and MAE cells expressing GATA-2 (K) (n = 3 independent experiments).
(L) RNA-seq of Gata2 and Gpr65 mRNA in FACS-sorted endothelial cells (EC), hemogenic endothelial cells (HEC), hematopoietic cells (HC), and hematopoietic stem cells (HSC) from the AGM (Solaimani Kartalaei et al., 2015).
Error bars represent SEM. ∗p < 0.05; ∗∗∗p < 0.001 (two-tailed unpaired Student's t test).
To discover GPCRs that control HSC generation and/or activity, we evaluated GPCR expression in the AGM. Of the 314 GPCRs annotated by RNA-seq, 85 were expressed at >5 transcripts per million (Figure 3C), a level that can be validated with high frequency by real-time RT-PCR. Of the 85 GPCRs, 20 were downregulated in the +9.5−/− AGM versus +9.5+/+ AGM (Figure 3D), indicating GATA-2-regulation. Using our previous microarray dataset (untreated or β-estradiol-treated G1E-ER-GATA-1 erythroid precursor cells) (DeVilbiss et al., 2013), we found that four of the 20 GATA-2-regulated GPCRs were GATA-1-regulated (Figure 3D). Our strategy refined the 314 non-olfactory, AGM-expressed GPCRs to yield Adora3, Gpr65, Ltb4r1, and P2ry1, which are GATA-2- and GATA-1-regulated.
A GATA Factor-GPCR Incoherent Feedforward Loop Suppresses AGM Hematopoiesis
As shared gene expression patterns can infer functional interconnectivity, we compared Adora3, Gpr65, Ltb4r1, P2ry1, and Gata2 expression patterns in mouse cells and tissues. Only Gpr65 resembled Gata2, both being expressed in HSPCs and mast cells (Figure S2) (http://biogps.org). As GATA-1 represses Gata2 during erythroid maturation via a GATA switch, Gata2 expression declines upon erythroid maturation. Mining the Erythron Database, which provides transcriptomics data during erythroid precursor cell maturation into erythrocytes (Kingsley et al., 2013), revealed Gata2 and Gpr65 repression upon erythroid differentiation. Gpr65 was the only one of the four GPCR genes to have a Gata2-like expression pattern (Figure 3E). To further compare expression patterns, we quantitated Gata2 and Gpr65 mRNA in G1E-ER-GATA-1 cells treated with β-estradiol to induce erythroid maturation and in FACS-sorted R1, R2, R3, and R4/5 fetal liver cell populations. Gpr65 and Gata2 were repressed during erythroid maturation (Figures 3F and 3G). These correlations are consistent with GATA-2 upregulating Gpr65 expression in the AGM and may point to a functional link between GATA-2 and GPR65. Chromatin immunoprecipitation (ChIP)-sequencing analysis in human (Figure 3H) and mouse (Figure 3I) cells revealed GATA-2 occupancy at Gpr65, suggesting that GATA-2 directly regulates Gpr65 transcription. Comparison of +9.5+/+ and +9.5−/− AGM revealed that reduced Gata2 expression in the +9.5−/− AGM decreased Gpr65 expression, and Gpr65 expression was undetectable in the yolk sac (Figure 3J). Previously, we demonstrated that GATA-2 expression in mouse aortic endothelial cells increases transcription of certain GATA-2 target genes (Katsumura et al., 2014). In this system, GATA-2 increased Gpr65 expression (Figure 3K), indicating that GATA-2 regulates Gpr65 expression in multiple contexts. To analyze the Gpr65 expression pattern in distinct cell types within the AGM, we mined RNA-seq data obtained with FACS-sorted endothelial cells, hemogenic endothelial cells, hematopoietic cells, and HSCs from the AGM (Solaimani Kartalaei et al., 2015). This analysis revealed that Gpr65 is detectable in all cell types, and the levels are lower in HSCs (Figure 3L).
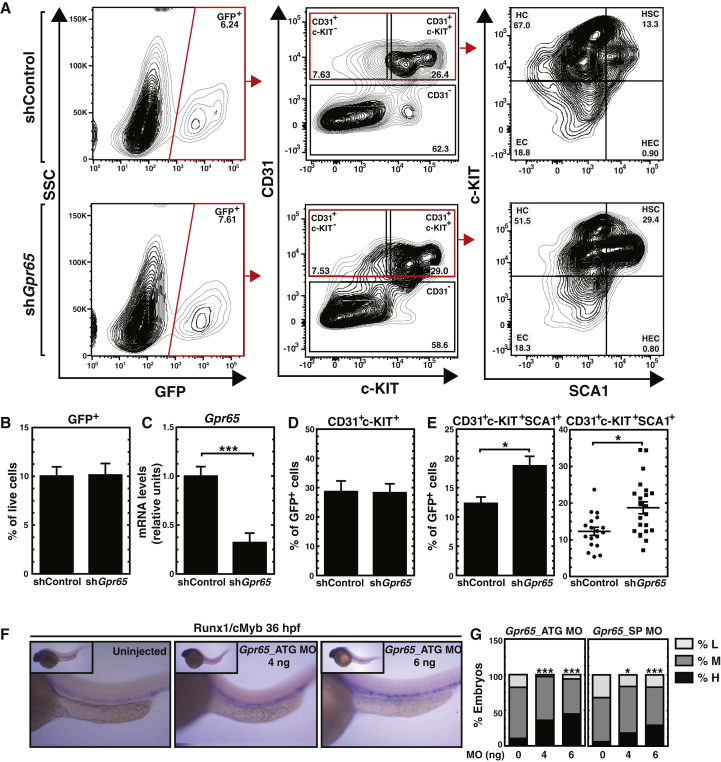
To test whether GPR65 controls hematopoiesis in the AGM, we conducted a loss-of-function analysis using a Gpr65 short hairpin RNA (shRNA) retrovirus. E11.5 AGMs were infected, and after culturing for 96 hr hematopoietic cell populations were quantitated by flow cytometry. Quantitation of GFP+ live cells indicated that control shRNA (shLuc) and shGpr65 retroviruses had an indistinguishable infection efficiency (Figures 4A and 4B). Gpr65 knockdown reduced Gpr65 mRNA by 60%–70% (Figure 4C). While downregulating Gpr65 did not alter CD31+c-KIT+ hematopoietic cells (Figures 4A and 4D), it increased CD31+c-KIT+SCA1+ HSC-containing, multipotent hematopoietic cells (Figures 4A and 4E). Retroviral-mediated Gpr65 shRNA expression did not alter CD31+c-KIT+ hematopoietic cells and CD31+c-KIT+SCA1+ HSC-containing cells in GFP− cells (Figure S3). These results indicate that GPR65 suppresses hematopoiesis in the AGM.
Figure 4.
GPR65 Suppresses Hematopoiesis in the Mouse and Zebrafish AGM
(A) Representative flow cytometric plots of CD31+c-KIT+ and CD31+c-KIT+SCA1+ cell populations in control or Gpr65 shRNA-treated AGMs after 96 hr of culture.
(B) Quantitation of GFP+ cells with total live cells (9 litters: shLuc [n = 22 embryos]; shGpr65 [n = 26 embryos]).
(C) RT-PCR analysis of Gpr65 mRNA levels in FACS-sorted GFP+ cells (6 litters: shLuc [n = 15 embryos]; shGpr65 [n = 15 embryos]).
(D and E) Analysis of flow cytometry data expressed as percentage of CD31+c-KIT+ (D) and CD31+c-KIT+Scal1+ (E) cells in GFP+ cells (D: 9 litters: shLuc [n = 22 embryos]; shGpr65 [n = 26 embryos]; E: 7 litters: shLuc [n = 18]; shGpr65 [n = 22]).
(F) Representative images of ISH with the HSPC markers Runx1/cMyb at 36 hr post-fertilization.
(G) Quantitation of ISH data expressed as percentage of embryos with high, medium, and low Runx1/cMyb staining in total embryos (ATG MO 0 ng [124 embryos]; ATG MO 4 ng [75 embryos]; ATG MO 6 ng [66 embryos]; SP MO 0 ng [97 embryos]; SP MO 4 ng [49 embryos]; SP MO 6 ng [58 embryos]). Gpr65_ATG MO: morpholino targeting the translation start site of Gpr65; Gpr65_SP MO: morpholino blocking the splicing of Gpr65.
Error bars represent SEM. ∗p < 0.05; ∗∗∗p < 0.001 (two-tailed unpaired Student's t test).
To assess whether Gpr65 activity to suppress hematopoiesis operates in other systems, we used a morpholino (MO) targeting the Gpr65 translation start site (Gpr65_ATG MO) to reduce Gpr65 expression in zebrafish embryos. The Gpr65_ATG MO was injected into 1-cell stage embryos, which were analyzed for expression of the HSPC markers Runx1/cmyb using in situ hybridization (ISH) at 36 hr post-fertilization. The Gpr65_ATG MO dose-dependently increased expression of the HSPC markers Runx1/cmyb in the embryos (Figures 4F and 4G). A second MO, which blocks Gpr65 splicing (Gpr65_SP MO), yielded an identical result; Gpr65 downregulation induced expression of the HSPC markers Runx1/cmyb (Figure 4G). Thus, GPR65 suppresses hematopoiesis in mouse and zebrafish embryos.
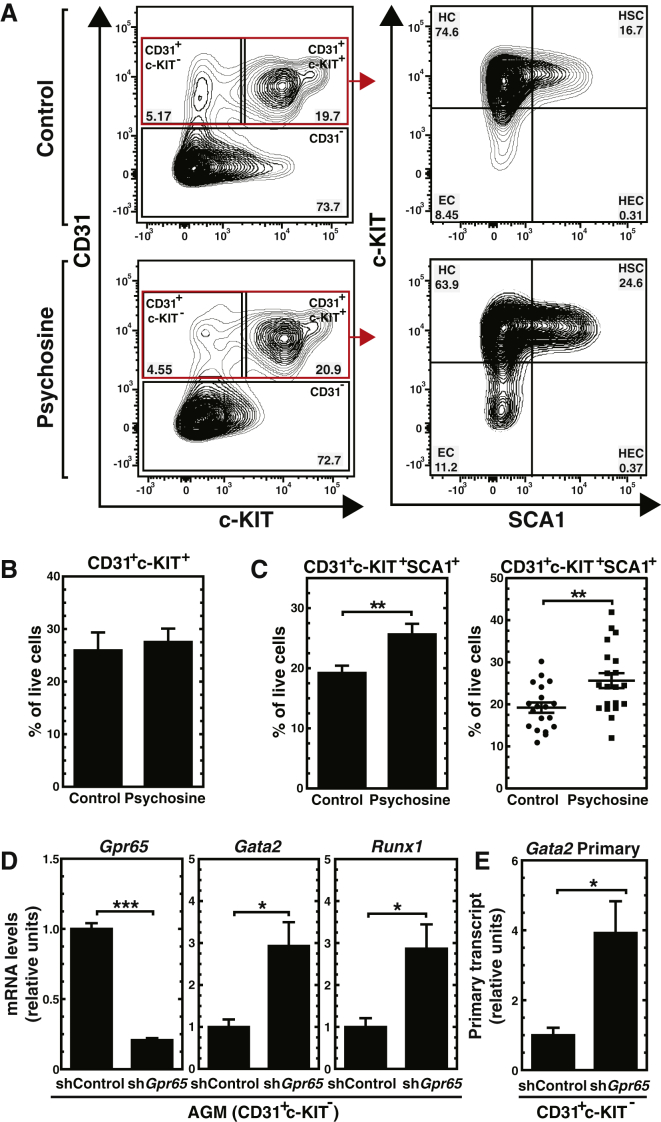
shRNA and MO-based loss-of-function strategies may be confounded by off-target effects. As an alternative strategy we used a GPR65 antagonist, the lysosphingolipid galactosylsphingosine (psychosine). Psychosine was initially proposed to be a GPR65 agonist, based on the GPR65 requirement for psychosine-induced multi-nuclear cell formation (Im et al., 2001). Subsequently, it was demonstrated that GPR65 is a proton-sensing receptor and that psychosine antagonizes GPR65 (Radu et al., 2005, Tobo et al., 2015, Wang et al., 2004). We treated E11.5 AGMs with vehicle or psychosine, and after 96 hr, hematopoietic cells were quantitated by flow cytometry. While psychosine did not alter CD31+c-KIT+ cells (Figures 5A and 5B), it increased the CD31+c-KIT+SCA1+ cell population, which is known to contain multipotent hematopoietic precursors (Figures 5A and 5C). In aggregate, the mouse and zebrafish studies indicate that GPR65 is an endogenous suppressor of hematopoiesis.
Figure 5.
Psychosine Promotes Hematopoiesis in the AGM and GPR65 Suppresses Hematopoiesis by Repressing Gata2 Expression
(A) Representative flow cytometric plots of CD31+c-KIT+ and CD31+c-KIT+SCA1+ cell populations in the AGM after 4 days of culture with 20 μM psychosine.
(B and C) The average percentage of CD31+c-KIT+ (B) and CD31+c-KIT+SCA1+ (C) cell populations with vehicle or psychosine treatment (6 litters: control [n = 19 embryos]; psychosine [n = 20 embryos]).
(D and E) RT-PCR analysis of Gpr65, Gata2, and Runx1 mRNA (D) and Gata2 primary transcript (E) in FACS-sorted CD31+c-KIT− cells (n = 3 independent experiments).
Error bars represent SEM. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 (two-tailed unpaired Student's t test).
GPR65 Establishes Repressive Chromatin and Disrupts an Activating Complex on a cis Element Required for Gata2 Transcription
To elucidate the mechanism underlying GPR65 suppression of hematopoiesis, we considered whether GPR65 might downregulate key regulators of HSC generation/activity. After knocking down GPR65, we isolated infected CD31+c-KIT− endothelial cells that give rise to HSCs. Downregulating Gpr65 mRNA by 60%–70% increased Gata2 mRNA 2.9-fold (p = 0.03) and its downstream target Runx1 mRNA 2.9-fold (p = 0.04) (Figure 5D). The knockdown elevated Gata2 primary transcripts 3.9-fold (p = 0.04) (Figure 5E), indicating that GPR65 suppresses Gata2 transcription.
To determine whether GPR65 regulates Gata2 expression in zebrafish, we analyzed Gata2 expression using ISH at 36 hr post-fertilization. Zebrafish have two Gata2 homologs; Gata2b is enriched in hemogenic endothelium and regulates HSPC emergence (Butko et al., 2015). Whereas the majority of uninjected embryos exhibited broad staining in the AGM, there was no clear linear zone enriched in hemogenic endothelium. However, Gpr65_ATG MO-injected embryos exhibited the linear zone (Figure S4), suggesting that GPR65 suppresses Gata2 expression in zebrafish embryos. Given that GATA-2 promotes HSC emergence in the AGM and regulates HSC activity (de Pater et al., 2013, Gao et al., 2013), we propose that GPR65 suppresses hematopoiesis by limiting Gata2 expression and GATA-2 levels.
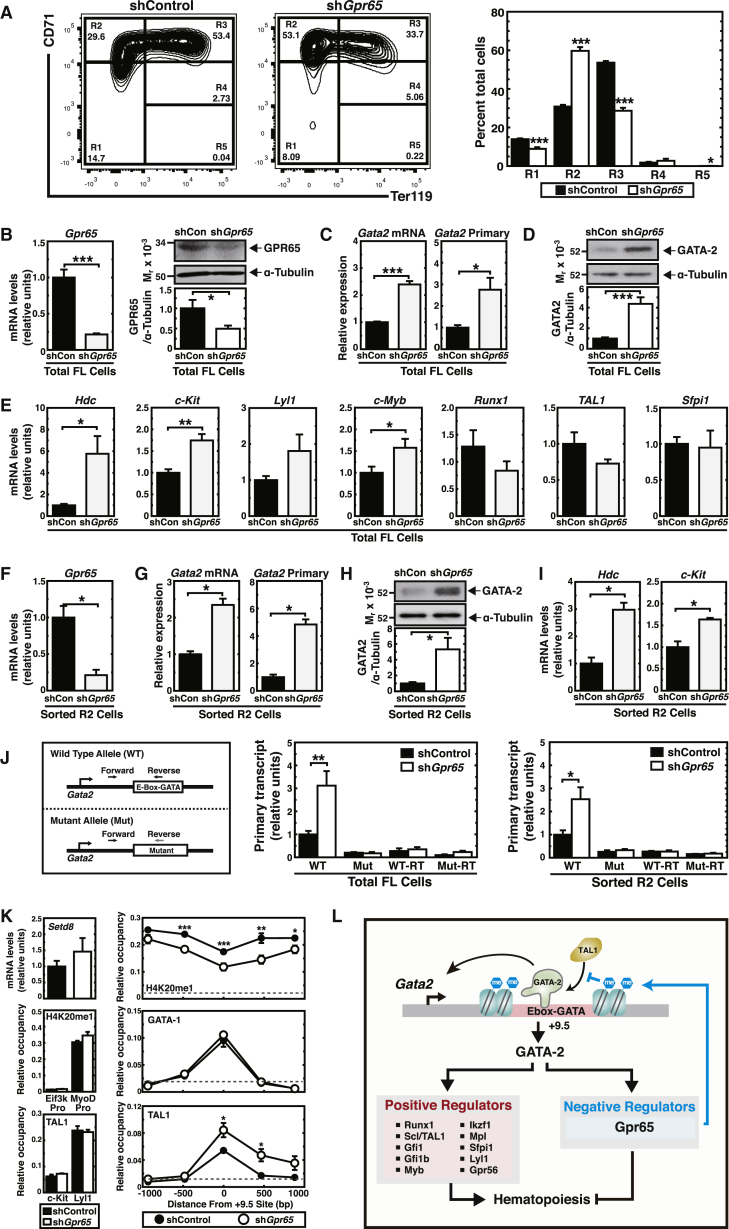
As Gpr65 is expressed in AGM endothelium and HSPCs, we asked whether GPR65 represses Gata2 expression in other contexts. Fetal liver hematopoietic precursors express Gata2, and as GATA-1 rises upon erythroid maturation, Gata2 is repressed (McIver et al., 2014). We isolated Lin− hematopoietic precursors from E14.5 fetal livers (McIver et al., 2014) and tested whether reducing Gpr65 expression with the Gpr65 shRNA retrovirus alters Gata2 expression. Cells were expanded for 72 hr to increase HSPCs, while suppressing differentiation. Downregulating Gpr65 increased the proerythroblast-enriched R2 population 1.9-fold (p = 0.0002) and reduced the R3 population (early and late basophilic erythroblasts) 1.6-fold (p = 0.0003) (Figure 6A). Increased R2 cells, concomitant with reduced R3 cells, suggests that GPR65 promotes erythroid maturation. Downregulating Gpr65 mRNA by 70%–80%, which lowered GPR65 protein by 50%, increased Gata2 mRNA and primary transcripts 2.4 (p = 3.65 × 10−6) and 2.8-fold (p = 0.014), respectively (Figures 6B and 6C). Western blot analysis of fetal liver cells revealed that reducing GPR65 expression upregulated GATA-2 (Figure 6D). Increased GATA-2 selectively elevated GATA-2 target gene expression (Figure 6E). To test whether increased Gata2 expression reflected a change in cellularity, we used FACS to isolate the GATA-2-expressing R2 population and compared gene expression in control and knockdown R2 cells. Gpr65 knockdown upregulated Gata2 mRNA 2.3-fold (p = 0.02), primary transcript 4.8-fold (p = 0.01), GATA-2 protein, and GATA-2 target genes in FACS-sorted R2 cells (Figures 6F–6I). These results indicate that GPR65 represses Gata2 in the AGM and fetal liver. Since GATA-1 needs to repress Gata2 transcription early in erythroid maturation, upregulated Gata2 expression caused by the Gpr65 knockdown would be expected to increase immature R2 cells as observed (Figure 6A).
Figure 6.
GPR65 Enhances H4K20me1 and Limits Scl/TAL1 Occupancy at the +9.5 Enhancer
(A) Representative flow cytometric plots of erythroid maturation based on CD71 and Ter119 expression 3 days after HSPC expansion. The average percentage of cells in R1 through R5 populations after infection with control or Gpr65 shRNA is depicted on the right (n = 3 independent experiments).
(B) RT-PCR analysis of Gpr65 mRNA in fetal liver cells (n = 5 independent experiments) (left). Western blot of GPR65 in fetal liver cells (top right), and quantification of GPR65 and α-tubulin intensities by densitometry (n = 8 independent experiments) (bottom right).
(C) RT-PCR analysis of Gata2 mRNA and primary transcript in total fetal liver cells (n = 5 independent experiments).
(D) Western blot of GATA-2 in fetal liver cells (top) and quantification of GATA-2/α-tubulin ratio by densitometry (n = 6 independent experiments) (bottom).
(E) RT-PCR analysis of GATA-2 target gene expression in fetal liver cells (n = 5 independent experiments).
(F–I) Real-time RT-PCR analysis of Gpr65 mRNA (F), Gata2 mRNA (G), Gata2 primary transcript (G), and GATA-2 target genes in FACS-sorted R2 cells (n = 3 independent experiments) (I). Western blot of GATA-2 in FACS-sorted R2 cells and quantification of intensities of GATA-2 to α-tubulin band by densitometric analysis (n = 4 independent experiments) (H).
(J) Fetal liver HSPCs were isolated from E14.5 embryos heterozygous for the +9.5 site at Gata2. Cells were infected with control or shGpr65 and expanded for 3 days (left). Allele-specific real-time RT-PCR analysis of Gata2 transcripts from the wild-type and mutant +9.5 alleles in total fetal liver cells (n = 3 independent experiments) (middle) and FACS-sorted R2 cells (n = 3 independent experiments) (right).
(K) RT-PCR analysis of Setd8 mRNA in fetal liver cells treated with control or Gpr65 shRNA (n = 5 independent experiments) (top left). H4K20me1, GATA-1, and Scl/TAL1 chromatin occupancy measured by quantitative ChIP at the +9.5 site in expanding fetal liver HSPCs treated with control or Gpr65 shRNA (H4K20me1: n = 8 independent experiments; GATA-1: n = 4 independent experiments; Scl/TAL1: n = 6 independent experiments) (right). H4K20me1 levels at the repressed MyoD promoter and the active Eif3k promoter (middle left). Scl/TAL1 chromatin occupancy 114 kb upstream from the c-Kit promoter and Lyl1 exon1 as controls (bottom left). The dashed line illustrates the highest value obtained with PI antibody.
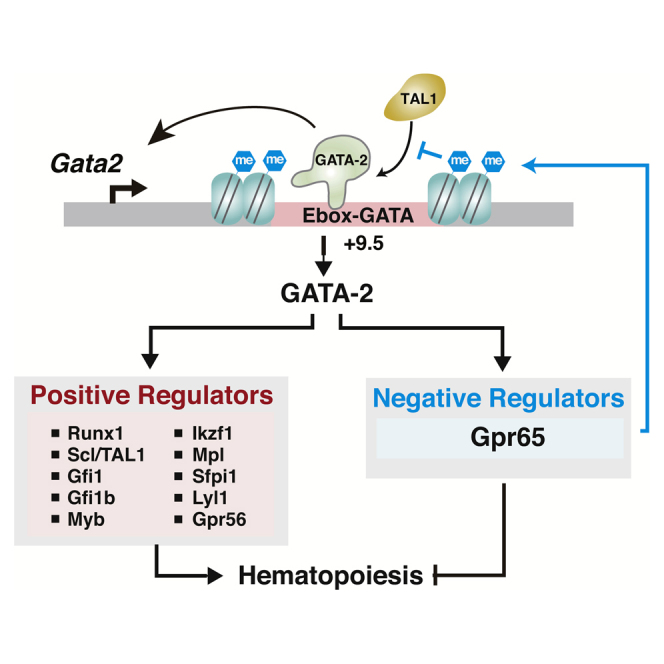
(L) GATA-2, a positive regulator of hematopoiesis, upregulates Gpr65, which encodes a negative regulator of hematopoiesis, to control HSC emergence. GPR65 represses Gata2 expression by increasing H4K20me1, which restricts +9.5 occupancy by the activator Scl/TAL1.
Error bars represent SEM. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 (two-tailed unpaired Student's t test).
The +9.5 enhances Gata2 expression in the AGM and fetal liver (Gao et al., 2013, Johnson et al., 2012, Sanalkumar et al., 2014). Deleting the +9.5 abrogated HSC generation in the AGM (Gao et al., 2013) and disrupted establishment of the HSPC compartment in the fetal liver (Johnson et al., 2012). To determine whether increased Gata2 transcription upon Gpr65 knockdown requires the +9.5, we isolated Lin− HSPCs from E14.5 + 9.5+/− fetal livers and infected cells with Gpr65 shRNA retrovirus. After 72 hr of expansion culture, allele-specific primers were used to quantitate primary transcripts from wild-type and mutant 9.5 alleles in fetal liver cells and FACS-purified R2 cells (Figure 6J). Gpr65 knockdown upregulated Gata2 primary transcripts from the wild-type, but not the +9.5 mutant, allele (Figure 6J), demonstrating importance of the +9.5 for Gata2 transcription.
Previously, we demonstrated that SetD8, the enzyme that monomethylates H4K20 (Beck et al., 2012), represses Gata2 expression via the +9.5 site (DeVilbiss et al., 2015). We tested the relevance of this mechanism to GPR65-mediated Gata2 repression. We conducted quantitative ChIP analysis for H4K20me1 in fetal liver cells (control or Gpr65 knockdown) after culturing for 72 hr. Downregulating Gpr65 did not alter the SetD8 mRNA level (Figure 6K). Gpr65 downregulation reduced H4K20me1 at the +9.5 site (p = 0.002) and at sites 480 bp upstream (p = 0.002), 466 bp downstream (p = 0.006), and 880 bp downstream (p = 0.02) of the +9.5 site (Figure 6K). H4K20me1 was unaltered at the repressed muscle-specific MyoD promoter and the constitutively active Eif3k promoter. These results support a mechanism in which GPR65 represses Gata2 by increasing a repressive chromatin mark at the +9.5 site. The basic helix-loop-helix transcription factor Scl/TAL1 activates Gata2 transcription through the +9.5 site, and Scl1/TAL1 chromatin occupancy is reduced at the +9.5 site during GATA-1-mediated Gata2 repression (Sanalkumar et al., 2014). As Gpr65 downregulation decreased H4K20me1 at the +9.5 site, this alteration may generate chromatin that is more accessible to cognate binding factors. To determine whether GPR65 alters GATA-1 and Scl/TAL1 occupancy, we quantitated GATA-1 and Scl/TAL1 occupancy at the +9.5 site in fetal liver cells infected with control shRNA or Gpr65 shRNA-expressing retrovirus. While knocking down Gpr65 did not alter GATA-1 occupancy at the +9.5, the knockdown increased Scl/TAL1 occupancy at the +9.5 1.6-fold (p = 0.02) but not at other target loci (Lyl1 and Kit) (Figures 6K and S5). These results suggest that GPR65 represses Gata2 by establishing repressive chromatin that limits Scl/TAL1 occupancy at the +9.5 site (Figure 6L).
Discussion
Genetic networks orchestrating stem and progenitor cell transitions can be deconstructed into regulatory modules termed network motifs (Alon, 2007, Shoval and Alon, 2010). Whereas major progress has been made to identify individual genes controlling hematopoiesis (Gao et al., 2013, Guo et al., 2013, Lara-Astiaso et al., 2014, Li et al., 2014, Moignard et al., 2013, Solaimani Kartalaei et al., 2015), many questions remain unanswered regarding how the genes form network motifs, how the network motifs amalgamate into circuitry, and whether the circuitry has an inherent plasticity and undergoes remodeling in states of altered hematopoiesis such as stress, aging, and malignancy.
Since the GATA-2-dependent genetic network promotes hematopoiesis (Gao et al., 2013, Solaimani Kartalaei et al., 2015), it is reasonable to infer that GATA-2-induced factors are positive mediators of key steps, including EHT, HSPC self-renewal, and differentiation. In erythroid cells, GATA-1 activates heme biosynthetic genes, globin subunits, and constituents of the red cell cytoskeleton (Cheng et al., 2009, Fujiwara et al., 2009, Welch et al., 2004, Yu et al., 2009), all required for erythroid maturation. Moreover, GATA-1 represses Gata2, Lyl1, and Kit (Grass et al., 2003, Johnson et al., 2007, Munugalavadla et al., 2005), to enable erythroid maturation. GATA-2 activates Kit (Jing et al., 2008), an essential regulator of HSPCs (Heissig et al., 2002, Huang et al., 1990), consistent with GATA-2 promoting HSPC genesis and function.
Using a coupled bioinformatics-experimental strategy, we analyzed the large GPCR gene family to discover GATA-2-induced GPCRs that promote HSPC transitions. This analysis led to the surprising finding that GATA-2 activates Gpr65 expression, which suppresses hematopoiesis via negative feedback on Gata2. This negative feedback mechanism conforms to a type I incoherent feedforward loop (Mangan and Alon, 2003), which is defined as an input (GATA-2) that elicits an output (EHT or hematopoiesis) through positive (increased Gpr65 expression) and negative (GPR65 suppression of hematopoiesis) paths. Type I incoherent feedforward loops shape the dynamics of a mechanism, with the feedforward loop conferring a pulse of activity to accelerate the reaction (Mangan and Alon, 2003). Since the constituents and network motifs governing HSPC transitions are still being identified, the dynamics of steps in these transitions are largely unexplored. The mechanism of GPR65-mediated Gata2 repression involved elevation of a repressive histone mark, H4K20me1, linked to reduced occupancy by the activator Scl/TAL1. GPR65 might directly target chromatin to restrict Scl/TAL1 occupancy or might reduce Scl/TAL1 occupancy prior to chromatin modification. Regardless of the order of events, our analysis established a GATA factor-GPCR-dependent type I incoherent feedforward loop that suppresses, rather than promotes hematopoiesis.
Gpr65, or T cell death-associated gene 8 (Tdag8), is a proton/acid-activated GPCR (Choi et al., 1996, Wang et al., 2004). Whereas GPR65 had not been linked to HSPC transitions, it was reported to be a pro-apoptotic factor in glucocorticoid-induced lymphocyte apoptosis (Malone et al., 2004) and to regulate cytokine production from macrophages (Mogi et al., 2009). Targeted deletion of Gpr65 revealed it was dispensable for glucocorticoid-induced thymocyte apoptosis (Radu et al., 2006). These results were suggested to reflect redundancy with related pH-sensing GPCRs, which would create an obstacle to dissect Gpr65 function in vivo. Although the impact of the pH-sensing mechanism to GATA-2 function in the AGM and fetal liver is unclear, the endogenous GPR65 antagonist psychosine resembled Gpr65 shRNA in upregulating Gata2 expression and hematopoiesis.
As GPR65 suppressed HSPC generation from mouse AGM explants, it is instructive to consider the consequences of ablating this mechanism in vivo. Abrogation of the mechanism would promote HSPC genesis and function. Since ablating Gpr65 would be expected to increase HSPCs, evaluating the role of the GATA-2-GPR65 circuit in vivo will require a careful quantitative analysis. Considerations of type I incoherent feedforward loops (Alon, 2007, Mangan and Alon, 2003) would infer an alteration of the dynamics of specific steps of hematopoiesis, which might not be evident from steady-state analyses. It is attractive to propose that this suppressive mechanism is vital when the demand for hematopoiesis increases, e.g. during stress, since an unopposed increase could yield deleterious blood cell elevations.
In summary, we discovered that GATA-2 activity to promote hematopoiesis involves induction of both positive and negative mediators, the balance of which establishes the physiological output. Based on the GPR65 pH-sensing function, it will be crucial to analyze the pH microenvironment of the AGM and other sites of hematopoiesis and to establish the role of GPR65 as a mediator of pH-dependent HSPC transitions.
Experimental Procedures
Mice and Embryo Generation
Gata2 +9.5-mutant mice were described by Johnson et al. (2012). Pregnant females were euthanized with CO2, and freshly isolated embryos were transferred into cold PBS for dissection. Animal experiments were carried out with ethical approval of the Association for the Assessment and Accreditation of Laboratory Animal Care at the University of Wisconsin-Madison.
AGM Explant Culture
AGMs were dissected from E11.5 embryos. AGMs were infected (see below) and cultured as described by Gao et al. (2013). Intact AGMs were cultured for 4 days on Durapore filters (Millipore) at the air-liquid interface in IMDM+ (Iscove's modified Dulbecco's medium) (Gibco) supplemented with 20% fetal bovine serum (FBS; Gemini), 4 mM L-glutamine (Gibco), 1% penicillin/streptomycin (Cellgro), 0.1 mM mercaptoethanol, 100 ng/ml interleukin-3 (R&D Systems), 100 ng/ml Flt3L (R&D), and 1.5% conditioned medium from a Kit ligand-producing Chinese hamster ovary cell line.
Retroviral Infection
Detailed methodology is presented in Supplemental Experimental Procedures. Gpr65 shRNA were cloned into the MSCV-PIG (IRES-GFP) vector (kindly provided by Dr. Mitchell Weiss) (McIver et al., 2014) using BglII and XhoI restriction sites. Retroviral expression vectors for Gata2 and shLuc were described by Katsumura et al. (2014). Retroviruses were packaged by co-transfecting 293T cells with pCL-Eco packaging vector. Retroviral supernatant was collected 24 and 48 hr post transfection and centrifuged to remove cells and debris. The Retro-X Concentrator (Clontech #631455) was used to concentrate retrovirus. E11.5 AGMs were infected with 20 μl concentrated retrovirus and 8 μg/ml polybrene in 500 μl explant culture media at 1,200 × g for 90 min at 30°C. AGMs were subjected to explant culture. Primary erythroid precursors were spinfected with 100 μl retrovirus supernatant and 8 μg/ml polybrene in 400 μl fetal liver expansion medium at 1,200 × g for 90 min at 30°C. After centrifugation, 500 μl pre-warmed fetal liver expansion medium was added, and cells were incubated at 37°C for 72 hr. Details for concentrating retrovirus and Gpr65 shRNA sequences are available in Supplemental Experimental Procedures.
Flow Cytometry
Detailed methodology is presented in Supplemental Experimental Procedures.
ChIP Assay
Detailed methodology is presented in Supplemental Experimental Procedures.
Zebrafish Morpholino Knockdown
Detailed methodology is presented in Supplemental Experimental Procedures.
Statistical Analysis
Student's t tests were conducted using GraphPad Software or Microsoft Excel. p Values in all figures are denoted by asterisks: ∗p < 0.05, ∗∗p < .01, and ∗∗∗p < .001.
Author Contributions
All authors participated in designing experiments and/or analyzing data. J.L.L. conducted the zebrafish experiments and analyzed the data. X.G. and E.H.B. wrote the manuscript in consultation with other authors.
Acknowledgments
The work was supported by NIH DK68634 and DK50107 (to E.H.B.), Cancer Center Support Grant P30CA014520, and NIH HL04880, HL032262, HL10001-05, and DK092760 (to L.I.Z). We thank Michelle Ammerman and Prathibha Sanalkumar for assistance with zebrafish experiments and Western blotting, respectively.
Published: February 18, 2016
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures and five figures and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2016.01.008.
Accession Numbers
The accession number for GATA-2, RUNX1, and FLI1 ChIP-seq in human CD34+ cells reported in the paper is GEO: GSE45144. The accession number for GATA-2 and TAL1 ChIP-seq in HPC7 cells reported in the paper is GEO: GSE22178. The accession number for GATA-2 ChIP-seq in Lin− BM cells reported in the paper is GEO: GSM641911. The accession number for GATA-2 ChIP-seq in G1E cells reported in the paper is GEO: GSM722387.
Supplemental Information
References
- Adams G.B., Alley I.R., Chung U.I., Chabner K.T., Jeanson N.T., Lo Celso C., Marsters E.S., Chen M., Weinstein L.S., Lin C.P. Haematopoietic stem cells depend on Galpha(s)-mediated signalling to engraft bone marrow. Nature. 2009;459:103–107. doi: 10.1038/nature07859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon U. Network motifs: theory and experimental approaches. Nat. Rev. Genet. 2007;8:450–461. doi: 10.1038/nrg2102. [DOI] [PubMed] [Google Scholar]
- Beck D.B., Oda H., Shen S.S., Reinberg D. PR-Set7 and H4K20me1: at the crossroads of genome integrity, cell cycle, chromosome condensation, and transcription. Genes Dev. 2012;26:325–337. doi: 10.1101/gad.177444.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck D., Thoms J.A., Perera D., Schutte J., Unnikrishnan A., Knezevic K., Kinston S.J., Wilson N.K., O'Brien T.A., Gottgens B. Genome-wide analysis of transcriptional regulators in human HSPCs reveals a densely interconnected network of coding and noncoding genes. Blood. 2013;122:e12–22. doi: 10.1182/blood-2013-03-490425. [DOI] [PubMed] [Google Scholar]
- Bertrand J.Y., Chi N.C., Santoso B., Teng S., Stainier D.Y., Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisset J.C., van Cappellen W., Andrieu-Soler C., Galjart N., Dzierzak E., Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H.E., Orschell C.M., Clapp D.W., Hangoc G., Cooper S., Plett P.A., Liles W.C., Li X., Graham-Evans B., Campbell T.B. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J. Exp. Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butko E., Distel M., Pouget C., Weijts B., Kobayashi I., Ng K., Mosimann C., Poulain F.E., McPherson A., Ni C.W. Gata2b is a restricted early regulator of hemogenic endothelium in the zebrafish embryo. Development. 2015;142:1050–1061. doi: 10.1242/dev.119180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Wu W., Kumar S.A., Yu D., Deng W., Tripic T., King D.C., Chen K.-B., Zhang Y., Drautz D. Erythroid GATA1 function revealed by genome-wide analysis of transcription factor occupancy, histone modifications, and mRNA expression. Genome Res. 2009;19:2172–2184. doi: 10.1101/gr.098921.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.W., Lee S.Y., Choi Y. Identification of a putative G protein-coupled receptor induced during activation-induced apoptosis of T cells. Cell Immunol. 1996;168:78–84. doi: 10.1006/cimm.1996.0051. [DOI] [PubMed] [Google Scholar]
- de Pater E., Kaimakis P., Vink C.S., Yokomizo T., Yamada-Inagawa T., van der Linden R., Kartalaei P.S., Camper S.A., Speck N., Dzierzak E. Gata2 is required for HSC generation and survival. J. Exp. Med. 2013;210:2843–2850. doi: 10.1084/jem.20130751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVilbiss A.W., Boyer M.E., Bresnick E.H. Establishing a hematopoietic genetic network through locus-specific integration of chromatin regulators. Proc. Natl. Acad. Sci. USA. 2013;110:E3398–E3407. doi: 10.1073/pnas.1302771110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVilbiss A.W., Sanalkumar R., Hall B.D., Katsumura K.R., de Andrade I.F., Bresnick E.H. Epigenetic determinants of erythropoiesis: role of the histone methyltransferase SetD8 in promoting erythroid cell maturation and survival. Mol. Cell Biol. 2015;35:2073–2087. doi: 10.1128/MCB.01422-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzierzak E., Speck N.A. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat. Immunol. 2008;9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., O'Geen H., Keles S., Blahnik K., Linnemann A.K., Kang Y.A., Choi K., Farnham P.J., Bresnick E.H. Discovering hematopoietic mechanisms through genome-wide analysis of GATA factor chromatin occupancy. Mol. Cell. 2009;36:667–681. doi: 10.1016/j.molcel.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Johnson K.D., Chang Y.I., Boyer M.E., Dewey C.N., Zhang J., Bresnick E.H. Gata2 cis-element is required for hematopoietic stem cell generation in the mammalian embryo. J. Exp. Med. 2013;210:2833–2842. doi: 10.1084/jem.20130733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling W., Allen R.S., Guan X., Jin P., Uchida N., Dovey M., Harris J.M., Metzger M.E., Bonifacino A.C., Stroncek D. Prostaglandin E2 enhances human cord blood stem cell xenotransplants and shows long-term safety in preclinical nonhuman primate transplant models. Cell Stem Cell. 2011;8:445–458. doi: 10.1016/j.stem.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass J.A., Boyer M.E., Pal S., Wu J., Weiss M.J., Bresnick E.H. GATA-1-dependent transcriptional repression of GATA-2 via disruption of positive autoregulation and domain-wide chromatin remodeling. Proc. Natl. Acad. Sci. USA. 2003;100:8811–8816. doi: 10.1073/pnas.1432147100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass J.A., Jing H., Kim S.-I., Martowicz M.L., Pal S., Blobel G.A., Bresnick E.H. Distinct functions of dispersed GATA factor complexes at an endogenous gene locus. Mol. Cell Biol. 2006;26:7056–7067. doi: 10.1128/MCB.01033-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiu J., Shimizu R., D'Altri T., Fraser S.T., Hatakeyama J., Bresnick E.H., Kageyama R., Dzierzak E., Yamamoto M., Espinosa L. Hes repressors are essential regulators of hematopoietic stem cell development downstream of Notch signaling. J. Exp. Med. 2013;210:71–84. doi: 10.1084/jem.20120993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G., Luc S., Marco E., Lin T.W., Peng C., Kerenyi M.A., Beyaz S., Kim W., Xu J., Das P.P. Mapping cellular hierarchy by single-cell analysis of the cell surface repertoire. Cell Stem Cell. 2013;13:492–505. doi: 10.1016/j.stem.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissig B., Hattori K., Dias S., Friedrich M., Ferris B., Hackett N.R., Crystal R.G., Besmer P., Lyden D., Moore M.A. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggatt J., Mohammad K.S., Singh P., Hoggatt A.F., Chitteti B.R., Speth J.M., Hu P., Poteat B.A., Stilger K.N., Ferraro F. Differential stem- and progenitor-cell trafficking by prostaglandin E2. Nature. 2013;495:365–369. doi: 10.1038/nature11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu A.P., Johnson K.D., Falcone E.L., Sanalkumar R., Sanchez L., Hickstein D.D., Cuellar-Rodriguez J., Lemieux J.E., Zerbe C.S., Bresnick E.H. GATA2 haploinsufficiency caused by mutations in a conserved intronic element leads to MonoMAC syndrome. Blood. 2013;121:3830–3837. doi: 10.1182/blood-2012-08-452763. S1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E., Nocka K., Beier D.R., Chu T.Y., Buck J., Lahm H.W., Wellner D., Leder P., Besmer P. The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell. 1990;63:225–233. doi: 10.1016/0092-8674(90)90303-v. [DOI] [PubMed] [Google Scholar]
- Im D.S., Heise C.E., Nguyen T., O'Dowd B.F., Lynch K.R. Identification of a molecular target of psychosine and its role in globoid cell formation. J. Cell Biol. 2001;153:429–434. doi: 10.1083/jcb.153.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing H., Vakoc C.R., Ying L., Mandat S., Wang H., Zheng X., Blobel G.A. Exchange of GATA factors mediates transitions in looped chromatin organization at a developmentally regulated gene locus. Mol. Cell. 2008;29:232–242. doi: 10.1016/j.molcel.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K.D., Boyer M.E., Kang J.A., Wickrema A., Cantor A.B., Bresnick E.H. Friend of GATA-1-independent transcriptional repression: a novel mode of GATA-1 function. Blood. 2007;109:5230–5233. doi: 10.1182/blood-2007-02-072983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K.D., Hsu A.P., Ryu M.J., Wang J., Gao X., Boyer M.E., Liu Y., Lee Y., Calvo K.R., Keles S. Cis-element mutated in GATA2-dependent immunodeficiency governs hematopoiesis and vascular integrity. J. Clin. Invest. 2012;122:3692–3704. doi: 10.1172/JCI61623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K.D., Kong G., Gao X., Chang Y.-I., Hewitt K.J., Sanalkumar R., Prathibha R., Ranheim E.A., Dewey C.N., Zhang J. Cis-regulatory mechanisms governing stem and progenitor cell transitions. Sci. Adv. 2015;1:e1500503. doi: 10.1126/sciadv.1500503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumura K.R., Yang C., Boyer M.E., Li L., Bresnick E.H. Molecular basis of crosstalk between oncogenic Ras and the master regulator of hematopoiesis GATA-2. EMBO Rep. 2014;15:938–947. doi: 10.15252/embr.201438808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley P.D., Greenfest-Allen E., Frame J.M., Bushnell T.P., Malik J., McGrath K.E., Stoeckert C.J., Palis J. Ontogeny of erythroid gene expression. Blood. 2013;121:e5–e13. doi: 10.1182/blood-2012-04-422394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerstrom M.C., Schioth H.B. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug Discov. 2008;7:339–357. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- Lara-Astiaso D., Weiner A., Lorenzo-Vivas E., Zaretsky I., Jaitin D.A., David E., Keren-Shaul H., Mildner A., Winter D., Jung S. Immunogenetics. Chromatin state dynamics during blood formation. Science. 2014;345:943–949. doi: 10.1126/science.1256271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Jothi R., Cui K., Lee J.Y., Cohen T., Gorivodsky M., Tzchori I., Zhao Y., Hayes S.M., Bresnick E.H. Nuclear adaptor Ldb1 regulates a transcriptional program essential for the maintenance of hematopoietic stem cells. Nat. Immunol. 2011;12:129–136. doi: 10.1038/ni.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Esain V., Teng L., Xu J., Kwan W., Frost I.M., Yzaguirre A.D., Cai X., Cortes M., Maijenburg M.W. Inflammatory signaling regulates embryonic hematopoietic stem and progenitor cell production. Genes Dev. 2014;28:2597–2612. doi: 10.1101/gad.253302.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Lan Y., Schwartz-Orbach L., Korol E., Tahiliani M., Evans T., Goll M.G. Overlapping requirements for Tet2 and Tet3 in Normal development and hematopoietic stem cell emergence. Cell Rep. 2015;12:1133–1143. doi: 10.1016/j.celrep.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling K.W., Ottersbach K., van Hamburg J.P., Oziemlak A., Tsai F.Y., Orkin S.H., Ploemacher R., Hendriks R.W., Dzierzak E. GATA-2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells. J. Exp. Med. 2004;200:871–882. doi: 10.1084/jem.20031556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Li D., Yu Y.Y., Kang I., Cha M.J., Kim J.Y., Park C., Watson D.K., Wang T., Choi K. Induction of hematopoietic and endothelial cell program orchestrated by ETS transcription factor ER71/ETV2. EMBO Rep. 2015;16:654–669. doi: 10.15252/embr.201439939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugus J.J., Chung Y.S., Mills J.C., Kim S.I., Grass J., Kyba M., Doherty J.M., Bresnick E.H., Choi K. GATA2 functions at multiple steps in hemangioblast development and differentiation. Development. 2007;134:393–405. doi: 10.1242/dev.02731. [DOI] [PubMed] [Google Scholar]
- Maeno M., Mead P.E., Kelley C., Xu R.H., Kung H.F., Suzuki A., Ueno N., Zon L.I. The role of BMP-4 and GATA-2 in the induction and differentiation of hematopoietic mesoderm in Xenopus laevis. Blood. 1996;88:1966–1972. [PubMed] [Google Scholar]
- Malone M.H., Wang Z., Distelhorst C.W. The glucocorticoid-induced gene tdag8 encodes a pro-apoptotic G protein-coupled receptor whose activation promotes glucocorticoid-induced apoptosis. J. Biol. Chem. 2004;279:52850–52859. doi: 10.1074/jbc.M408040200. [DOI] [PubMed] [Google Scholar]
- Mangan S., Alon U. Structure and function of the feed-forward loop network motif. Proc. Natl. Acad. Sci. USA. 2003;100:11980–11985. doi: 10.1073/pnas.2133841100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May G., Soneji S., Tipping A.J., Teles J., McGowan S.J., Wu M., Guo Y., Fugazza C., Brown J., Karlsson G. Dynamic analysis of gene expression and genome-wide transcription factor binding during lineage specification of multipotent progenitors. Cell Stem Cell. 2013;13:754–768. doi: 10.1016/j.stem.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIver S.C., Kang Y.A., DeVilbiss A.W., O'Driscoll C.A., Ouellette J.N., Pope N.J., Camprecios G., Chang C.J., Yang D., Bouhassira E.E. The exosome complex establishes a barricade to erythroid maturation. Blood. 2014;124:2285–2297. doi: 10.1182/blood-2014-04-571083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi C., Tobo M., Tomura H., Murata N., He X.D., Sato K., Kimura T., Ishizuka T., Sasaki T., Sato T. Involvement of proton-sensing TDAG8 in extracellular acidification-induced inhibition of proinflammatory cytokine production in peritoneal macrophages. J. Immunol. 2009;182:3243–3251. doi: 10.4049/jimmunol.0803466. [DOI] [PubMed] [Google Scholar]
- Moignard V., Macaulay I.C., Swiers G., Buettner F., Schutte J., Calero-Nieto F.J., Kinston S., Joshi A., Hannah R., Theis F.J. Characterization of transcriptional networks in blood stem and progenitor cells using high-throughput single-cell gene expression analysis. Nat. Cell Biol. 2013;15:363–372. doi: 10.1038/ncb2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munugalavadla V., Dore L.C., Tan B.L., Hong L., Vishnu M., Weiss M.J., Kapur R. Repression of c-kit and its downstream substrates by GATA-1 inhibits cell proliferation during erythroid maturation. Mol. Cell Biol. 2005;25:6747–6759. doi: 10.1128/MCB.25.15.6747-6759.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North T.E., Goessling W., Walkley C.R., Lengerke C., Kopani K.R., Lord A.M., Weber G.J., Bowman T.V., Jang I.H., Grosser T. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S.H., Zon L.I. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radu C.G., Nijagal A., McLaughlin J., Wang L., Witte O.N. Differential proton sensitivity of related G protein-coupled receptors T cell death-associated gene 8 and G2A expressed in immune cells. Proc. Natl. Acad. Sci. USA. 2005;102:1632–1637. doi: 10.1073/pnas.0409415102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radu C.G., Cheng D., Nijagal A., Riedinger M., McLaughlin J., Yang L.V., Johnson J., Witte O.N. Normal immune development and glucocorticoid-induced thymocyte apoptosis in mice deficient for the T-cell death-associated gene 8 receptor. Mol. Cell Biol. 2006;26:668–677. doi: 10.1128/MCB.26.2.668-677.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Moreno A., Guiu J., Ruiz-Herguido C., Lopez M.E., Ingles-Esteve J., Riera L., Tipping A., Enver T., Dzierzak E., Gridley T. Impaired embryonic haematopoiesis yet normal arterial development in the absence of the Notch ligand Jagged1. EMBO J. 2008;27:1886–1895. doi: 10.1038/emboj.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues N.P., Janzen V., Forkert R., Dombkowski D.M., Boyd A.S., Orkin S.H., Enver T., Vyas P., Scadden D.T. Haploinsufficiency of GATA-2 perturbs adult hematopoietic stem cell homeostasis. Blood. 2005;106:477–484. doi: 10.1182/blood-2004-08-2989. [DOI] [PubMed] [Google Scholar]
- Roth B.L., Kroeze W.K. Integrated approaches for genome-wide interrogation of the druggable non-olfactory g protein-coupled receptor superfamily. J. Biol. Chem. 2015;290:19471–19477. doi: 10.1074/jbc.R115.654764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y., Kaneda K., Suekane A., Ichihara E., Nakahata S., Yamakawa N., Nagai K., Mizuno N., Kogawa K., Miura I. Maintenance of the hematopoietic stem cell pool in bone marrow niches by EVI1-regulated GPR56. Leukemia. 2013;27:1637–1649. doi: 10.1038/leu.2013.75. [DOI] [PubMed] [Google Scholar]
- Sanalkumar R., Johnson K.D., Gao X., Boyer M.E., Chang Y.I., Hewitt K.J., Zhang J., Bresnick E.H. Mechanism governing a stem cell-generating cis-regulatory element. Proc. Natl. Acad. Sci. USA. 2014;111:E1091–E1100. doi: 10.1073/pnas.1400065111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoval O., Alon U. SnapShot: network motifs. Cell. 2010;143:326-e1. doi: 10.1016/j.cell.2010.09.050. [DOI] [PubMed] [Google Scholar]
- Solaimani Kartalaei P., Yamada-Inagawa T., Vink C.S., de Pater E., van der Linden R., Marks-Bluth J., van der Sloot A., van den Hout M., Yokomizo T., van Schaick-Solerno M.L. Whole-transcriptome analysis of endothelial to hematopoietic stem cell transition reveals a requirement for Gpr56 in HSC generation. J. Exp. Med. 2015;212:93–106. doi: 10.1084/jem.20140767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y., Narumiya S. Prostaglandin E receptors. J. Biol. Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- Tobo A., Tobo M., Nakakura T., Ebara M., Tomura H., Mogi C., Im D.S., Murata N., Kuwabara A., Ito S. Characterization of imidazopyridine compounds as negative allosteric modulators of proton-sensing GPR4 in extracellular acidification-induced responses. PLoS One. 2015;10:e0129334. doi: 10.1371/journal.pone.0129334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripic T., Deng W., Cheng Y., Vakoc C.R., Gregory G.D., Hardison R.C., Blobel G.A. SCL and associated protein distinguish active from repressive GATA transcription factor complexes. Blood. 2008;113:2191–2201. doi: 10.1182/blood-2008-07-169417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompouki E., Bowman T.V., Lawton L.N., Fan Z.P., Wu D.C., DiBiase A., Martin C.S., Cech J.N., Sessa A.K., Leblanc J.L. Lineage regulators direct BMP and Wnt pathways to cell specific programs during differentiation and regeneration. Cell. 2011;147:577–589. doi: 10.1016/j.cell.2011.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai F.Y., Keller G., Kuo F.C., Weiss M., Chen J., Rosenblatt M., Alt F.W., Orkin S.H. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- Wang J.Q., Kon J., Mogi C., Tobo M., Damirin A., Sato K., Komachi M., Malchinkhuu E., Murata N., Kimura T. TDAG8 is a proton-sensing and psychosine-sensitive G-protein-coupled receptor. J. Biol. Chem. 2004;279:45626–45633. doi: 10.1074/jbc.M406966200. [DOI] [PubMed] [Google Scholar]
- Welch J.J., Watts J.A., Vakoc C.R., Yao Y., Wang H., Hardison R.C., Blobel G.A., Chodosh L.A., Weiss M.J. Global regulation of erythroid gene expression by transcription factor GATA-1. Blood. 2004;104:3136–3147. doi: 10.1182/blood-2004-04-1603. [DOI] [PubMed] [Google Scholar]
- Wilson N.K., Foster S.D., Wang X., Knezevic K., Schutte J., Kaimakis P., Chilarska P.M., Kinston S., Ouwehand W.H., Dzierzak E. Combinatorial transcriptional control in blood stem/progenitor cells: genome-wide analysis of ten major transcriptional regulators. Cell Stem Cell. 2010;7:532–544. doi: 10.1016/j.stem.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Wozniak R.J., Keles S., Lugus J.J., Young K., Boyer M.E., Tran T.T., Choi K., Bresnick E.H. Molecular hallmarks of endogenous chromatin complexes containing master regulators of hematopoiesis. Mol. Cell Biol. 2008;28:6681–6694. doi: 10.1128/MCB.01061-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Riva L., Xie H., Schindler Y., Moran T.B., Cheng Y., Yu D., Hardison R., Weiss M.J., Orkin S.H. Insights into GATA-1-mediated gene activation versus repression via genome-wide chromatin occupancy analysis. Mol. Cell. 2009;36:682–695. doi: 10.1016/j.molcel.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.