Abstract
Background
Recurrent aphthous stomatitis (RAS) is a recurrent painful ulcerative disorder that commonly affects the oral mucosa. Local and systemic factors such as trauma, food sensitivity, nutritional deficiencies, systemic conditions, immunological disorders and genetic polymorphisms are associated with the development of the disease. Helicobacter pylori (H. pylori) is a gram-negative, microaerophile bacteria, that colonizes the gastric mucosa and it was previously suggested to be involved in RAS development. In the present paper we reviewed all previous studies that investigated the association between RAS and H. pylori.
Material and Methods
A search in Pubmed (MEDLINE) databases was made of articles published up until July 2015 using the following keywords: Helicobacter Pylori or H. pylori and RAS or Recurrent aphthous stomatitis.
Results
Fifteen experimental studies that addressed the relationship between infection with H. pylori and the presence of RAS and three reviews, including a systematic review and a meta-analysis were included in this review. The studies reviewed used different methods to assess this relationship, including PCR, nested PCR, culture, ELISA and urea breath test. A large variation in the number of patients included in each study, as well as inclusion criteria and laboratorial methods was observed. H. pylori can be detected in the oral mucosa or ulcerated lesion of some patients with RAS. The quality of the all studies included in this review was assessed using levels of evidence based on the University of Oxford’s Center for Evidence Based Medicine Criteria.
Conclusions
Although the eradication of the infection may affect the clinical course of the oral lesions by undetermined mechanisms, RAS ulcers are not associated with the presence of the bacteria in the oral cavity and there is no evidence that H. pylori infection drives RAS development.
Key words:Campylobacter, elisa, h. pylori, Helicobacter Pylori, RAS, recurrent aphthous stomatitis, PCR.
Introduction
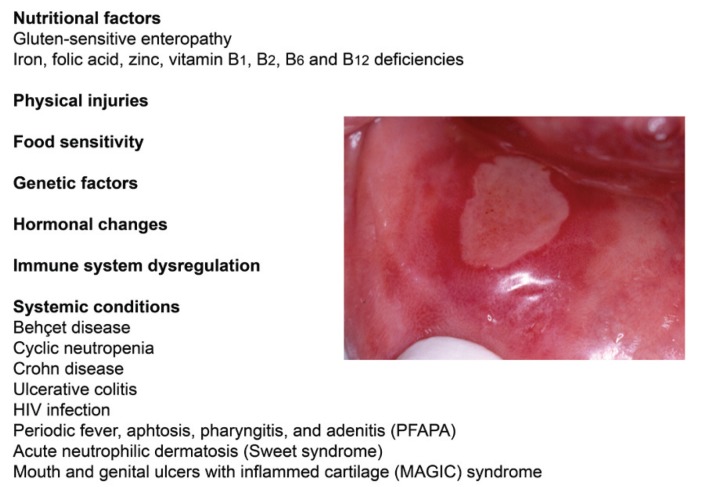
Recurrent aphthous stomatitis (RAS) is a very common condition characterized by solitary or multiple small, round, recurrent oral ulcers, with erythematous haloes and circumscribed margins. The appearance of the painful ulcers is periodic and the onset is usually during childhood and tends to diminish in severity with age (1). The diagnosis of RAS is based on clinical grounds but the etiology and pathogenesis remain unclear (2). Local and systemic factors have been suggested to affect the development of RAS. These factors are illustrated in the figure 1. For example, some genetic polymorphisms are associated with the occurrence of RAS (3). Some predisposing factors include trauma, hormonal changes, diet, nutritional deficiencies, Coeliac disease, and immunological disorders (4,5). Regarding nutritional deficiencies, some studies have found decreased levels of iron, vitamin B3 and B12, vitamin C, and folic acid (2).
Figure 1.
Clinical picture of a RAS lesion and the etiological factors associated with its development.
Helicobacter pylori (H. pylori) is a gram-negative, microaerophile bacteria, that colonizes the gastric mucosa and its infection is associated with the development of peptic ulcers, gastric mucosa associated lymphoid tissue lymphoma, and gastric cancer (6). Although H. pylori infection has been suggested to be one of the etiological factors in the pathogenesis of RAS, this association is debatable. In the present paper we review this issue and present the available evidence regarding this controversial topic.
Material and Methods
- Association between RAS and helicobacter pylori
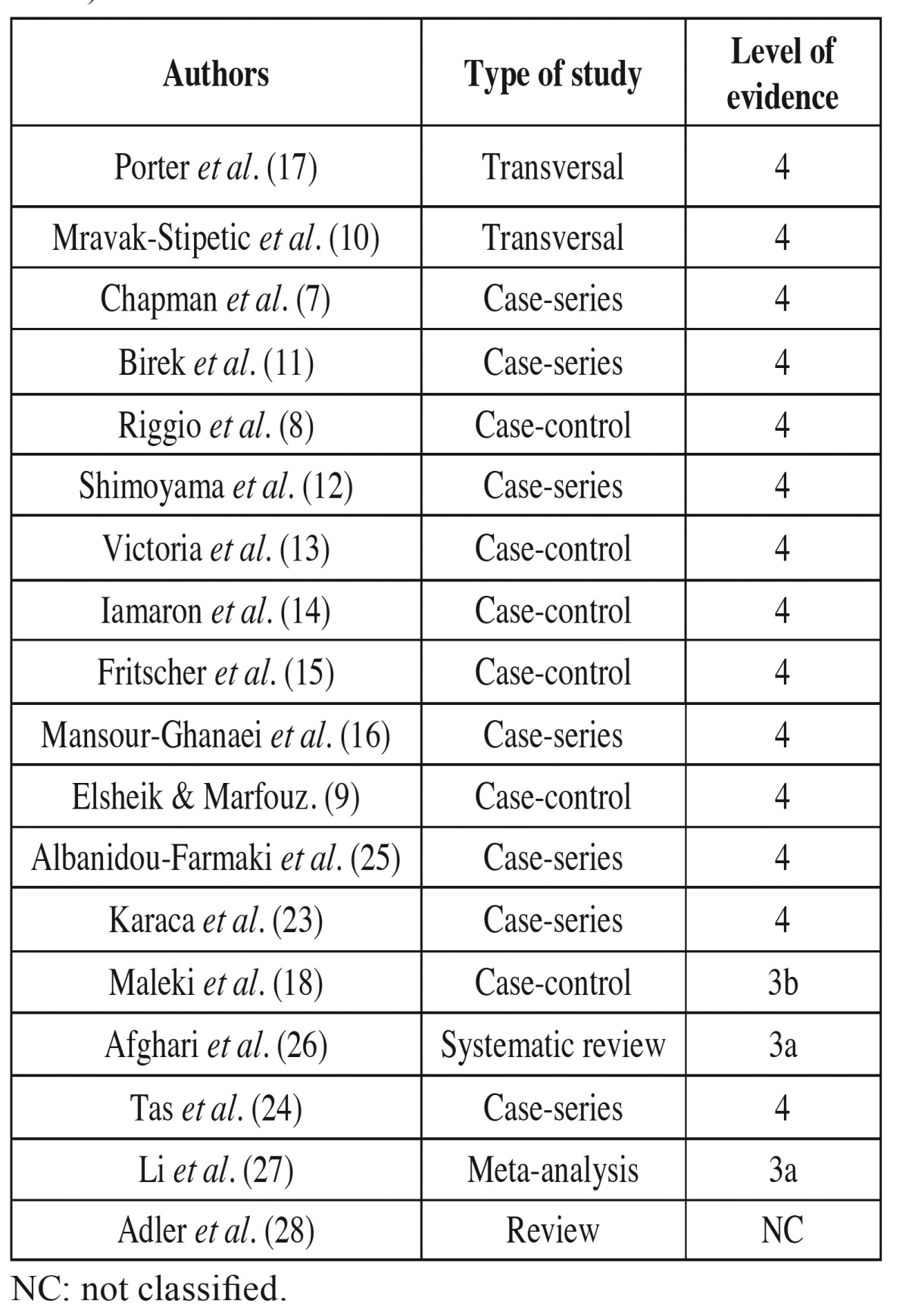
In this review, a search in Pubmed (MEDLINE) databases was made of articles published up until July 2015 using the following keywords: Helicobacter Pylori or H. pylori and RAS or Recurrent aphthous stomatitis. We included experimental and review studies that assessed the relationship between H. pylori and RAS. Quality of the studies was assessed using levels of evidence based on the University of Oxford’s Center for Evidence Based Medicine Criteria (CEMB 2009) ( Table 1).
Table 1. Classification of the studies selected for review according to type of study and level of evidence (CEMB 2009).
Results and Discussion
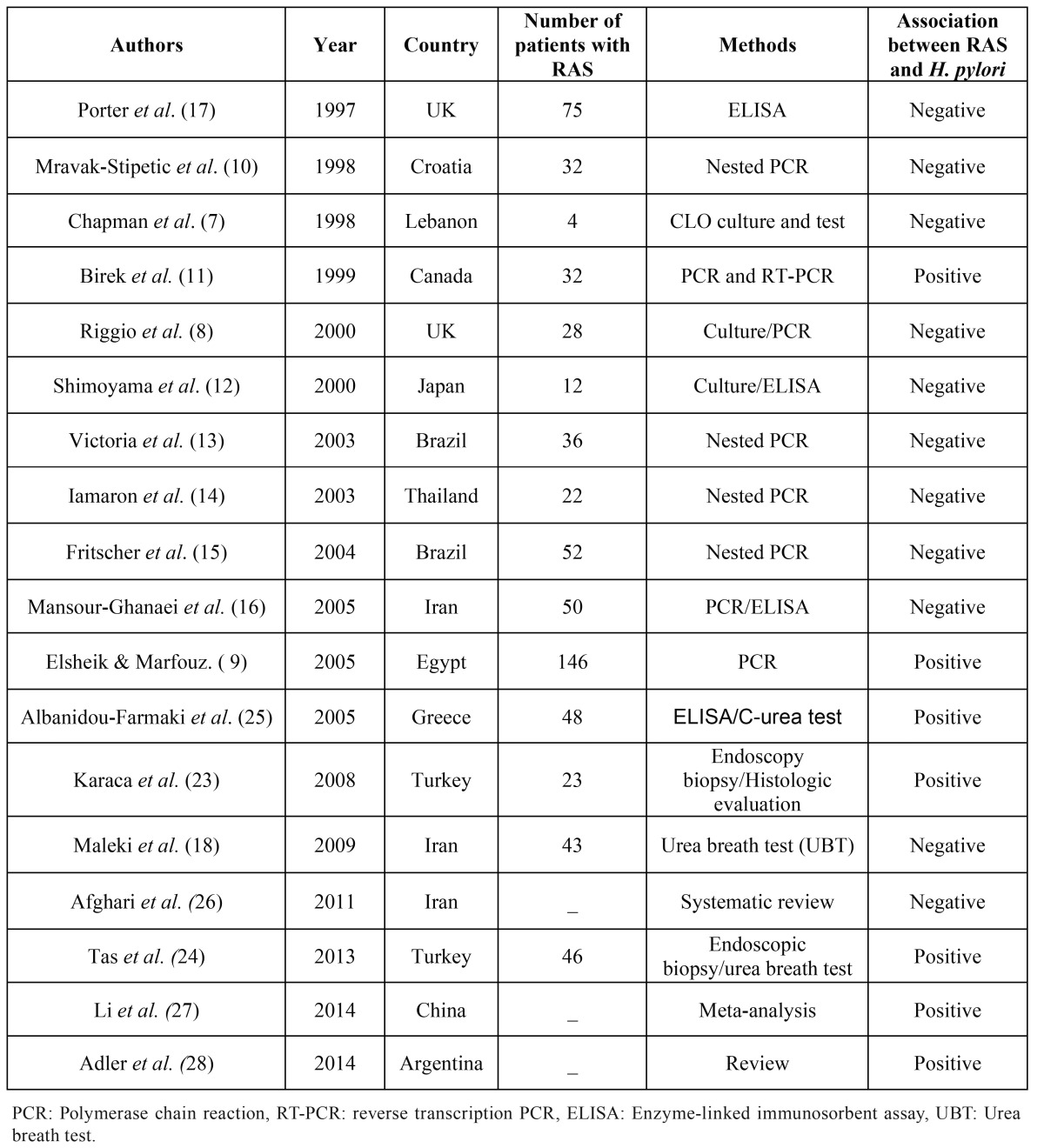
We included in this review fifteen experimental studies that addressed the relationship between infection with H. pylori and the presence of RAS and three reviews, including a systematic review and a meta-analysis that assessed this association ( Table 2). As shown in Table 2 there was a large variation in the number of patients evaluated in each study as well as the methods used to collect the samples or to identify H. pylori. While in some studies biopsies of the lesions were used (7-9), others used swabs (10-16). Ten out of fifteen studies did not demonstrate a statistically significant association between H. pylori and the presence of RAS (7,8,10,12-18). Another important variation that affects the analysis of the studies is the inclusion criteria used to diagnose RAS. As the histopathological features of RAS are nonspecific and the diagnosis is based on clinical grounds, the standardization of patients’ selection in future studies is important. There are good reviews about the clinical and diagnostic aspects of the disease (19).
Table 2. Studies that investigated the association between recurrent aphthous stomatitis (RAS) development and H. pylori infection.
The polymerase chain reaction (PCR) method was used to identify the presence of H. pylori DNA in eight studies (8-11,13-16). In two of them, the authors reported a statistically significant association between H.pylori presence and RAS (9,11). While Birek et al. (11) detected H. pylori in 72% of RAS samples using PCR and RT-PCR, Elsheikh & Mahfouz (9) reported that this was mainly observed in lesions localized in mucosa-associated lymphoid tissue of pharynx. However, it is necessary to emphasise that the simple detection of the bacteria in the oral lesion does not mean a causal relationship, as the microorganism may be a “passenger” and may not be the initiating factor of the disease. Most of the studies that employed PCR or nested PCR did not find association between the presence of the bacteria in the oral lesions and its development (8,10,13-16). H. pylori DNA was detected between 2% and 38.9% of RAS lesions included in the studies (8,10,13-16). None of these studies reported a statistically significant difference between the number of positive samples in the case and control groups. It is interesting that all authors who used the highly sensitive nested PCR method to detect the presence of H. pylori DNA in oral lesions did not find a positive relationship (10,13-15). The frequency of H. pylori in the non-affected oral mucosa of patients with RAS is not different from those without this condition (10,13-15).
Song et al. (20) compared three of the most used sets of primers for PCR analysis of H. pylori on samples of dental plaque. They concluded that the primer pairs EHC-U/EHC-L was the most recommended for the detection of H. pylori in the oral cavity. An important issue that should be addressed is that the PCR-based studies used different primers, some of which specific to conserved regions of the H. pylori genome, and some detecting the variable regions of the genome. The possible presence of other Helicobacter-like species in the mouth such as Campylobacter Rectus, Campylobacter Curvus and Campylobacter Concisus that are periodontopathogens and have up to 90% similarity with H. pylori is also a factor that needs to be considered (21). In addition, some of the primers used in the studies could also amplify other Helicobacter species that have also been found in human gut, such as H. fennelliae, H cinaedias (22). Thus appropriate positive and negative controls together with DNA direct sequencing of the PCR product are necessary to define the best PCR conditions and primers that should be used to detect H. pylori in samples collected from the oral cavity.
In two studies, patients with RAS were submitted to endoscopy biopsy to detect H. pylori (23,24). Both studies showed a positive relationship between the presence of the bacteria in the stomach and the occurrence of RAS in the mouth. In the studies of Karaca et al. (23) and Tas et al. (24) 87% and 65% of the patients with RAS, respectively, showed the bacteria in the gastric mucosa.
In four studies, the enzyme-linked immunosorbant assay (ELISA) was used to detect specific antibodies to H. pylori in RAS patients (12,16,17,25). In the study of Farmaki et al. (25) most of the patients with RAS were H. pylori positive in the ELISA test of serum and saliva. The other studies that performed ELISA did not find association between the presence of anti-H. pylori antibodies and RAS. Mansour-Ghanaei et al. (16) found that 26 (52%) of 50 subjects with RAS analysed were positive for H. pylori in the ELISA test. Of these, 16 patients had gastric disorders and 10 did not. These authors did not find a relationship between H. pylori positivity in the ELISA test and the presence of this bacterium in RAS lesions. In the study of Porter et al. (17) the frequency of anti-H. pylori seropositivity was not significantly greater in patients with RAS (30.6%) compared with patients with other ulcerated lesions (33.0%) and controls (24%). Shimoyama et al. (12) measured the presence of IgG antibody against H. pylori in the serum of 12 patients and found only three seropositive cases. Although no temporal analysis was performed in any of these studies, on the basis of these ELISA-based findings, there is no sound evidence of any association between the H. pylori infection and RAS.
Two studies used culture to detect H. pylori in RAS lesions. Shimoyama et al. (12) employed culture in samples collected by swabbing in the ulcer surface of 12 patients to detect H. pylori. Their results showed that none of 12 patients were positive for the bacteria in the culture. Chapman et al. (7) did not find the presence of the H. pylori in the biopsy samples obtained from patients with active RAS and history of RAS after performing CLO (Campylobacter-like organism) culture. In addition, no association between H. pylori and RAS was observed by Maleki et al. (18) using the UBT (Urea Breath Test). Furthermore, it is important to state that H. pylori in the oral cavity might be in a non-culturable coccoid state without the productive infection (12).
Recent literature review, including a systematic review and a meta-analysis, assessed the association between the infection by H. pylori and RAS. Afghari et al. (26) after reviewing nine publications up to 2011, concluded that there is no association. Li et al. (27) conducted a meta-analysis on studies published up to 2013 that evaluated the prevalence of infection by H. pylori in patients with RAS and controls. In this review they found an increased risk of RAS in patients with infection by H. pylori and eradication of this infection may prevent occurrence of RAS. Adler et al. (28) in a revision concluded that H. pylori infection in the occurrence of RAS would be associated with the anemia produced by H pylori-positive stomach disease.
- Helicobacter pylori eradication and RAS
While there is no evidence that RAS ulcers are directly associated with the infection by H. pylori, some studies have demonstrated that the eradication of the bacteria affects the clinical course of the oral disease (23-25). Karaca et al. (23) studied 23 patients with RAS and performed endoscopy and gastric biopsies. The patients with H. pylori were put on an eradication therapy and follow-up for annual recurrence. They observed significant positive effect of eradication on the recurrence rate, number, diameter, and amelioration of time of RAS. Albanidou-Farmaki et al. (25) studied 34 RAS patients that were positive for H. pylori. After the eradication therapy, the group of patients who had become negative showed a remarkable improvement with respect to recurrence of RAS lesions and symptom intensity. Tas et al. (24) studied forty-six patients with RAS during 6 months and recorded vitamin B12 levels. Thirty of these 46 subjects were positive for H. pylori. They found that vitamin B12 levels were significantly increased in the group of H. pylori-eradicated RAS patients. In addition, the number of RAS lesions in these patients decreased significantly. This study suggests that vitamin B12 levels could be the underlying mechanism that explains the effect of H. pylori eradication on RAS development. However, these findings need to be further confirmed in a large group of patients with a long follow-up period. Furthermore, other biological mechanisms related to H. pylori infection and treatment should be investigated.
Conclusion
The H. pylori can be occasionally detected in RAS lesions and the eradication of the infection may affect the clinical course of RAS lesions by undetermined mechanisms. However, most of the studies do not support the association of RAS ulcers with the presence of the bacteria in the oral cavity and the presence of the bacteria in the ulcer may reflect a passenger infection and not the trigger event. There is no convincing evidence of a direct cause- consequence effect of H. pylori infection and RAS ulcers development. This association requires further investigation by well-design prospective studies.The debate goes on.
Acknowledgments
CC Gomes and RS Gomez are research fellows at CNPq (National Counsil of Technological and Scientific Development), Brazil.
References
- 1.Scully C, Gorsky M, Lozada-Nur F. The diagnosis and management of recurrent aphthous stomatitis: a consensus approach. J Am Dent Assoc. 2003;134:200–07. doi: 10.14219/jada.archive.2003.0134. [DOI] [PubMed] [Google Scholar]
- 2.Chavan M, Jain H, Diwan N, Khedkar S, Shete A, Durkar S. Recurrent aphthous stomatitis: a review. J Oral Pathol Med. 2012;41:577–83. doi: 10.1111/j.1600-0714.2012.01134.x. [DOI] [PubMed] [Google Scholar]
- 3.Guimaraes AL, Correia-Silva Jde F, Sa AR, Victória JM, Diniz MG, Costa Fde O. Investigation of functional gene polymorphisms IL-1beta, IL-6, IL-10 and TNF-alpha in individuals with recurrent aphthous stomatitis. Arch Oral Biol. 2007;52:268–72. doi: 10.1016/j.archoralbio.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 4.McCullough MJ, Abdel-Hafeth S, Scully C. Recurrent aphthous stomatitis revisited; clinical features, associations, and new association with infant feeding practices? J Oral Pathol Med. 2007;36:615–20. doi: 10.1111/j.1600-0714.2007.00589.x. [DOI] [PubMed] [Google Scholar]
- 5.Porter S, Scully C. Aphthous ulcers (recurrent) Clin Evid. 2005;13:1687–94. [PubMed] [Google Scholar]
- 6.Gatta L, Vakil N, Vaira D, Scarpignato C. Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy. BMJ. 2013;347:f4587. doi: 10.1136/bmj.f4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman MS, Cimis RJ. Baughman RD. Lack of association between aphthous ulcers and Helicobacter pylori. Arch Dermatol. 1998;134:1634–5. doi: 10.1001/archderm.134.12.1634-a. [DOI] [PubMed] [Google Scholar]
- 8.Riggio MP, Lennon A, Wray D. Detection of Helicobacter pylori DNA in recurrent aphthous stomatitis tissue by PCR. J Oral Pathol Med. 2000;29:507–13. doi: 10.1034/j.1600-0714.2000.291005.x. [DOI] [PubMed] [Google Scholar]
- 9.Elsheikh MN, Mahfouz ME. Prevalence of Helicobacter pylori DNA in recurrent aphthous ulcerations in mucosa-associated lymphoid tissues of the pharynx. Arch Otolaryngol Head Neck Surg. 2005;131:804–8. doi: 10.1001/archotol.131.9.804. [DOI] [PubMed] [Google Scholar]
- 10.Mravak-Stipetic M, Gall-Troselj K, Lukac J, Kusic Z, Pavelic K, Pavelic J. Detection of Helicobacter pylori in various oral lesions by nested polymerase chain reaction (PCR) J Oral Pathol Med. 1998;27:1–3. doi: 10.1111/j.1600-0714.1998.tb02081.x. [DOI] [PubMed] [Google Scholar]
- 11.Birek C, Grandhi R, McNeill K, Singer D, Ficarra G, Bowden G. Detection of Helicobacter pylori in oral aphthous ulcers. J Oral Pathol Med. 1999;28:197–203. doi: 10.1111/j.1600-0714.1999.tb02024.x. [DOI] [PubMed] [Google Scholar]
- 12.Shimoyama T, Horie N, Kato T, Kaneko T, Komiyama K. Helicobacter pylori in oral ulcerations. J Oral Sci. 2000;42:225–9. doi: 10.2334/josnusd.42.225. [DOI] [PubMed] [Google Scholar]
- 13.Victoria JM, Kalapothakis E, Silva JF, Gomez RS. Helicobacter pylori DNA in recurrent aphthous stomatitis. J Oral Pathol Med. 2003;32:219–23. doi: 10.1034/j.1600-0714.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- 14.Iamaroon A, Chaimano S, Linpisarn S, Pongsiriwet S, Phornphutkul K. Detection of Helicobacter pylori in recurrent aphthous ulceration by nested PCR. J Oral Sci. 2003;45:107–10. doi: 10.2334/josnusd.45.107. [DOI] [PubMed] [Google Scholar]
- 15.Fritscher AM, Cherubini K, Chies J, Dias AC. Association between Helicobacter pylori and recurrent aphthous stomatitis in children and adolescents. J Oral Pathol Med. 2004;33:129–32. doi: 10.1111/j.0904-2512.2004.00074.x. [DOI] [PubMed] [Google Scholar]
- 16.Mansour-Ghanaei F, Asmar M, Bagherzadeh AH, Ekbataninezhad S. Helicobacter pylori infection in oral lesions of patients with recurrent aphthous stomatitis. Med Sci Monit. 2005;11:CR576–9. [PubMed] [Google Scholar]
- 17.Porter SR, Barker GR, Scully C, Macfarlane G, Bain L. Serum IgG antibodies to Helicobacter pylori in patients with recurrent aphthous stomatitis and other oral disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:325–8. doi: 10.1016/s1079-2104(97)90237-7. [DOI] [PubMed] [Google Scholar]
- 18.Maleki Z, Sayyari AA, Alavi K, Sayyari L, Baharvand M. A study of the relationship between Helicobacter pylori and recurrent aphthous stomatitis using a urea breath test. J Contemp Dent Pract. 2009;10:9–16. [PubMed] [Google Scholar]
- 19.Scully C, Porter S. Oral mucosal disease: recurrent aphthous stomatitis. Br J Oral Maxillofac Surg. 2008;46:198–206. doi: 10.1016/j.bjoms.2007.07.201. [DOI] [PubMed] [Google Scholar]
- 20.Song Q, Haller B, Schmid RM, Adler G, Bode G. Helicobacter pylori in dental plaque: a comparison of different PCR primer sets. Dig Dis Sci. 1999;44:479–84. doi: 10.1023/a:1026680618122. [DOI] [PubMed] [Google Scholar]
- 21.Silva DG, Tinoco EM, Rocha GA, Rocha AM, Guerra JB, Saraiva IE. Helicobacter pylori transiently in the mouth may participate in the transmission of infection. Mem Inst Oswaldo Cruz. 2010;105:657–60. doi: 10.1590/s0074-02762010000500009. [DOI] [PubMed] [Google Scholar]
- 22.Totten PA, Fennell CL, Tenover FC, Wezenberg JM, Perine PL, Stam WE. Campylobacter cinaedi (sp. nov.) and Campylobacter fennelliae (sp. nov.): two new Campylobacter species associated with enteric disease in homosexual men. J Infect Dis. 1985;151:131–9. doi: 10.1093/infdis/151.1.131. [DOI] [PubMed] [Google Scholar]
- 23.Karaca S, Seyhan M, Senol M, Harputluoglu MM, Ozcan A. The effect of gastric Helicobacter pylori eradication on recurrent aphthous stomatitis. Int J Dermatol. 2008;47:615–7. doi: 10.1111/j.1365-4632.2008.03667.x. [DOI] [PubMed] [Google Scholar]
- 24.Tas DA, Yakar T, Sakalli H, Serin E. Impact of Helicobacter pylori on the clinical course of recurrent aphthous stomatitis. J Oral Pathol Med. 2013;42:89–94. doi: 10.1111/j.1600-0714.2012.01197.x. [DOI] [PubMed] [Google Scholar]
- 25.Albanidou-Farmaki E, Giannoulis L, Markopoulos A, Fotiades S, Aggouridaki X, Farmakis K. Outcome following treatment for Helicobacter pylori in patients with recurrent aphthous stomatitis. Oral Dis. 2005;11:22–6. doi: 10.1111/j.1601-0825.2004.01053.x. [DOI] [PubMed] [Google Scholar]
- 26.Afghari P, Khazaei S, Kazemi S, Savabi O, Keshteli AH, Adibi P. The role of Helicobacter pylori in the development of recurrent aphthous stomatitis: SEPAHAN systematic review no. 9. Dent Res J (Isfahan) 2011;8(Suppl 1):S2–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Li L, Gu H, Zhang G. Association between recurrent aphtous stomatitis and Helicobacter pylori infection: a meta-analysis. Clin Oral Investg. 2014;18:1553–60. doi: 10.1007/s00784-014-1230-5. [DOI] [PubMed] [Google Scholar]
- 28.Adler I, Muiño A, Aguas S, Arada L, Dias M, Lence A. Helicobacter Pylori and oral pathology: Relathionship with the Gastric Infection. World J Gastroenterol. 2014;20:9922–35. doi: 10.3748/wjg.v20.i29.9922. [DOI] [PMC free article] [PubMed] [Google Scholar]