Abstract
Background
Advanced biliary tract carcinomas are associated with a poor prognosis, and palliative chemotherapy has only modest benefit. This multi-centre phase II study was conducted to determine the efficacy of capecitabine in combination with oxaliplatin in patients with inoperable gall bladder or biliary tract cancer.
Methods
This was a Phase II, non-randomised, two-stage Simon design, multi-centre study. Ethics approval was sought and obtained by the North West MREC, and then locally by the West Glasgow Hospitals Research Ethics Committee. Eligible patients with inoperable locally advanced or metastatic adenocarcinoma of the gall bladder or biliary tract and with adequate performance status, haematologic, renal, and hepatic function were treated with capecitabine (1000 mg/m2 po, twice daily, days 1–14) and oxaliplatin (130 mg/m2 i.v., day 1) every 3 weeks for up to six cycles. The primary objective of the study was to determine the objective tumour response rates (complete and partial). The secondary objectives included assessment of toxicity, progression-free survival, and overall survival.
Results
Forty-three patients were recruited between July 2003 and December 2005. The regimen was well tolerated with no grade 3/4 neutropenia or thrombocytopenia. Grade 3/4 sensory neuropathy was observed in six patients. Two-thirds of patients received their chemotherapy without any dose delays. Overall response rate was 23.8 % (95 % CI 12.05–39.5 %). Stable disease was observed in a further 13 patients (31 %) and progressive disease observed in 12 (28.6 %) of patients. The median progression-free survival was 4.6 months (95 % CI 2.8–6.4 months; Fig. 1) and the median overall survival 7.9 months (95 % CI 5.3–10.4 months; Fig. 2).
Conclusion
Capecitabine combined with oxaliplatin has a lower disease control and shorter overall survival than the combination of cisplatin with gemcitabine which has subsequently become the standard of care in this disease. However, capecitabine in combination with oxaliplatin does have modest activity in this disease, and can be considered as an alternative treatment option for patients in whom cisplatin and/or gemcitabine are contra-indicated.
Keywords: Gall bladder, Biliary tract, Capecitabine, Oxaliplatin
Background
Carcinomas of the gall bladder and biliary system are relatively rare malignancies accounting for approximately 4–5 % of all gastrointestinal cancers in Europe and the USA. Patients with these tumours often present with advanced disease, and so curative surgical options are limited and survival rates poor. Numerous cytotoxic agents have been evaluated both as single agent therapy and combination chemotherapy regimens (reviewed in [1]), but response rates in excess of 30 % have been difficult to achieve [1]. Nevertheless, chemotherapy can result in a significantly improved survival and an improved quality of life compared with best supportive care [2].
One of the most extensively studied cytotoxic agents in biliary tract cancer is 5-fluorouracil (5-FU), either alone or in combination. Response rates of 10–24 % have been reported for 5-FU as single agent [3–6], and of 10–40 % when used in combination [1], including 40 % with the ECF regimen, although most of these studies have been in small numbers of patients. In addition, there were no significant differences between single agent 5-FU and the FAM combination regimen in terms of median time to disease progression or median survival in randomised trials [7]. At the time of conducting this study, no standard therapy existed for advanced gall bladder or biliary tract cancer, with gemcitabine monotherapy or the combination of cisplatin with 5-FU among the most widely used regimens outside of clinical trials. However, the response rate for cisplatin in combination with 5-FU is only approximately 30–35 %. Consequently, further clinical trials of novel chemotherapy agents are warranted in gall bladder and biliary tract cancer.
Capecitabine is an oral fluoropyrimidine pro-drug with preferential conversion to 5-FU in tumour tissue compared to normal by exploiting the increased intra-tumoural expression of thymidine phosphorylase [8], and which is now extensively used in the treatment of colon [9–15], breast [16, 17] and gastric cancers [18]. Oxaliplatin is a third-generation cisplatin analogue, with activity and toxicity profiles that differ from those of other platinum derivatives, including carboplatin and cisplatin [19] and with clinical activity, either alone or in combination with 5-FU, in advanced colorectal cancer [9, 11–14, 20].
When the combination of capecitabine with oxaliplatin was evaluated in the phase I study, [12] a patient with gall bladder carcinoma who had progressed through treatment with a combination of 5-FU and leucovorin, had a partial response when treated with XELOX. This, taken together with the activity of single-agent 5-FU and of 5-FU in combination with cisplatin in biliary tract cancer, led us to explore the activity of a combination of capecitabine and oxaliplatin as a first-line therapy regimen within a phase II study in biliary tract cancers..
Patients and methods
Study design
This was a Phase II, non-randomised, two-stage Simon design, multi-centre study of capecitabine and oxaliplatin combination chemotherapy in patients with inoperable locally advanced or metastatic adenocarcinoma of the gall bladder or biliary tract. The primary objective of the study was to determine the objective tumour response rates (complete and partial), and secondary objectives included assessment of toxicity, progression-free survival, and overall survival. The study was approved by the North West MREC (Multi-Centre Research Ethics Committee) and then by the local research committees and NHS R&D departments of each participating institution. All patients gave written, informed, consent prior to any study related procedure.
Eligibility criteria
Eligible patients were those with histologically or cytologically confirmed adenocarcinoma of the gall bladder or biliary tract, with inoperable disease as determined by radiological assessment, laparotomy or laparoscopy. All patients had measurable disease, as defined by the response evaluation criteria in solid tumours criteria [RECIST] [21], with ECOG performance status ≤2, were at least 18 years of age, and had adequate renal (serum creatinine ≤1.5× institution’s upper limit of normal [ULN]), hepatic (bilirubin ≤2.5× ULN; transaminases ≤5× ULN), and haematological (absolute neutrophil count [ANC] ≥ 1500/mm3; platelet counts ≥ 100,000/mm3; haemoglobin ≥ 10 g/dl) function.
Patients were excluded if they had received any prior chemotherapy for metastatic disease, if they had received any radiotherapy, hormonal or immunotherapy within 4 weeks prior to study entry, or if they were unlikely to tolerate treatment. Other exclusion criteria included patients who were pregnant or of child-bearing potential and unwilling to use an acceptable method of birth control, peripheral sensory neuropathy of >grade 1 of any aetiology, known hypersensitivity to fluoropyrimidines or oxaliplatin, and any evidence of uncontrolled cardiac disease or any other serious medical or psychiatric disorder that would be a contra-indication for prescribing this chemotherapy regimen. Patients were also excluded if they will unable to reliably tolerate and comply with oral medication or if they had a lack of physical integrity of the GI tract leading to a malabsorption syndrome or intestinal obstruction that would impair administration or absorption of oral therapy.
Treatment administration
Capecitabine was administered in an intermittent schedule (14 days’ treatment; 7 days’ rest period) at a dose of 1000 mg/m2 twice daily orally in a 21-day treatment cycle. The two daily doses of capecitabine were administered 12 ± 2 h apart, within 30 min after a meal with approximately 200 ml of water. The total daily dose was “rounded-up” and given in equally divided twice daily doses. Patients were provided with a study diary card to record drug administration.
Oxaliplatin was administered on day 1 at a dose of 130 mg/m2 as a 2-hour intravenous infusion, after the morning dose of capecitabine, in a 21-day treatment schedule. If patients developed acute, laryngo-pharyngeal dysesthesiae, the subsequent doses of oxaliplatin were administered as a 6-h infusion.
Treatment was repeated every 21 days for a maximum of six courses, but was discontinued prior to this if there was evidence of disease progression, intolerable toxicity despite dose modification, patient refusal, or investigator decision to discontinue study therapy. Patients received prophylactic anti-emetic medication prior to the oxaliplatin infusion with intravenous dexamethasone and granisetron, followed by oral dexamethasone and domperidone for 3 and 5 days respectively.
Dose delays and dose modifications
Administration of subsequent courses of capecitabine and oxaliplatin was delayed for one week if non-haematological toxicity had not resolved to baseline levels or to ≤grade 1, or if the neutrophil count was <1.0 × 109/l or if the platelets were <75 × 109/l. Chemotherapy could be delayed for a maximum of 2 weeks to allow recovery from toxicity. If toxicity did not resolve after a delay of 2 weeks, then treatment was discontinued. Capecitabine was interrupted in patients who developed ≥grade 2 diarrhoea, stomatitis, or hand-foot syndrome, and doses of capecitabine omitted for toxicity were not replaced or restored. When toxicities had resolved to grade ≤1, capecitabine was recommenced with an appropriate dose modification as follows: a reduction of the daily dose by 25 % for first occurrence of grade 3 toxicity or second occurrence of grade 2 toxicity; a reduction of the daily dose by 50 % for grade 4 toxicity (first occurrence), grade 3 toxicity (second occurrence), or grade 2 toxicity (third occurrence). Capecitabine was discontinued for grade 4 toxicity (second occurrence), grade 3 toxicity (third occurrence), and grade 2 toxicity (fourth occurrence).
Supportive care
Palliative and supportive care was permitted during the study, but patients who required radiotherapy during the study were considered to have disease progression and were withdrawn from the study. Patients were not permitted to receive any other anti-cancer therapy during the study (including hormonal agents and immunotherapy). The use of prophylactic granulocyte colony stimulating factor (G-CSF) was not permitted during the first two cycles of drug administration.
Patient assessments
Patient assessments included clinical evaluation, toxicity assessments (NCI CTC version 2.0) and laboratory assessments every 3 weeks. Disease status was evaluated prior to starting chemotherapy and after three cycles of chemotherapy. Patients with stable disease or who were responding could receive a maximum of six cycles of chemotherapy, following which disease status was re-assessed within 4 weeks of the last cycle of chemotherapy. Disease assessment was by chest X-ray and CT scan of the abdomen, and other radiological assessments were performed as appropriate. Response was determined using RECIST [21]. Overall survival (all causes) was determined from the start of chemotherapy to the time of death and progression-free survival measured from the start of chemotherapy until subsequent disease progression.
Statistical analyses
As responses have been observed with 5-FU, with capecitabine and with the combination of capecitabine with oxaliplatin within the phase 1 study, a Gehan design for this phase II study was not appropriate. Therefore using a two-stage Simon design, it was calculated that recruitment of a total of 43 patients would give 80 % power at the 5 % significance level of detecting a response rate of ≥40 %, at which point it would be appropriate to consider further studies with this regimen, and a response rate of ≤20 %, below which this regimen would not be pursued in subsequent studies. Thirteen patients would be recruited in the first stage; if three or fewer responses were observed during this stage then recruitment would be halted and no further investigation of the combination warranted. If 13 or more responses were observed by the end of the study the combination would merit further development.
Results
Forty-three patients were recruited from six centres between July 2003 and December 2005. One patient was subsequently excluded from the analysis as they had a CVA and not received any treatment within the trial. Patient and tumour characteristics are shown in Table 1.
Table 1.
Patient characteristics
| Age, median (years) | 60.5 (range 37–81) |
| Gender | |
| Male | 24 (57.1) |
| Female | 18 (42.9) |
| ECOG performance status | |
| 0 | 9 (21.4) |
| 1 | 27 (64.3) |
| 2 | 6 (14.3) |
| Primary tumour | |
| Gallbladder | 18 |
| Bile ducts | 22 |
| Ampulla of vater | 2 |
| Metastatic disease at presentation | |
| Yes | 39 |
| No | 3 |
A total of 158 courses of chemotherapy were administered in 42 patients (median = 3). Eleven patients (26.2 %) completed all six planned courses of chemotherapy. All analyses were performed on an intention to treat basis.
Toxicity assessments
Haematological toxicity was minimal with no grade 3/4 neutropenia or thrombocytopenia, and only one occurrence of grade 3/4 anaemia. Grade 3/4 biochemical abnormalities (worst grade per patient, all cycles) included elevated alkaline phosphatase (10), bilirubin (3), glucose (3), and ALT, urea, and low albumin (all 1 each). Sensory neuropathy was observed in 37 patients, and was grade 3/4 in 6 patients. Other grade 3/4 toxicities included fatigue (6 patients), vomiting (5), nausea (4), diarrhoea (6), abdominal cramps (2), and anorexia (1) (maximum grade per patient).
Dose delays and modifications
Twenty-five patients (59.5 %) received chemotherapy without any dose delays. A total of 25 cycles of chemotherapy were delayed for thrombocytopenia (3 cycles), neutropenia (1), and deranged liver function (1 cycle). Other reasons for dose delays (21 cycles) included laryngeal dysaethesia, diarrhoea, hypokalaemia, chest infection, spinal cord compression, DVT, a requirement for paracentesis, abdominal pain, biliary sepsis, pulmonary embolism and elective change of biliary stent.
Dose modification of capecitabine was required for toxicity in 21 patients and of oxaliplatin in 6 patients. The duration of infusion of oxaliplatin had to be prolonged in 5 patients for pharyngo- laryngeal dysaesthesia.
Efficacy
With the exception of the patient who suffered a cerebrovascular event (CVA) and did not start treatment, all 42 patients were included in the efficacy analyses (intention–to–treat). There was one complete response (2.4 %) and 9 patients (21.4 %) had a partial response for an overall response rate of 23.8 % (95 % CI 12.05–39.5 %). Stable disease was observed in a further 13 (31 %) of patients, giving a disease control rate of 54.8 % (CR, PR and SD), with progressive disease observed in 12 (28.6 %) of patients (Table 2). Seven patients (16.7 %) did not have formal repeat assessments of disease status to determine response. Of these, five patients were withdrawn early due to treatment-related toxicities, one patient withdrew consent following the first cycle of treatment, and 1 patient died prior to cycle 2.
Table 2.
Response assessment
| Assessment of overall response in target and non-target lesions | Number of patients | % |
|---|---|---|
| CR | 1 | 2.4 |
| PR | 9 | 21.4 |
| SD | 13 | 31.0 |
| PD | 12 | 28.6 |
| Unevaluable, target lesions not assessed | 7 | 16.7 |
| Total | 42 | 100 |
The median progression-free survival is 4.6 months (95 % CI 2.8–6.4 month; Fig. 1) and the median overall survival is 7.9 months (95 % CI 5.3–10.4 months; Fig. 2).
Fig. 1.
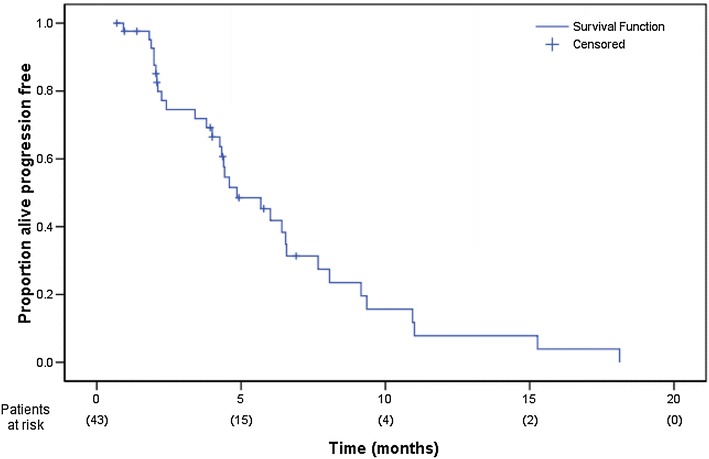
Progression-free survival
Fig. 2.
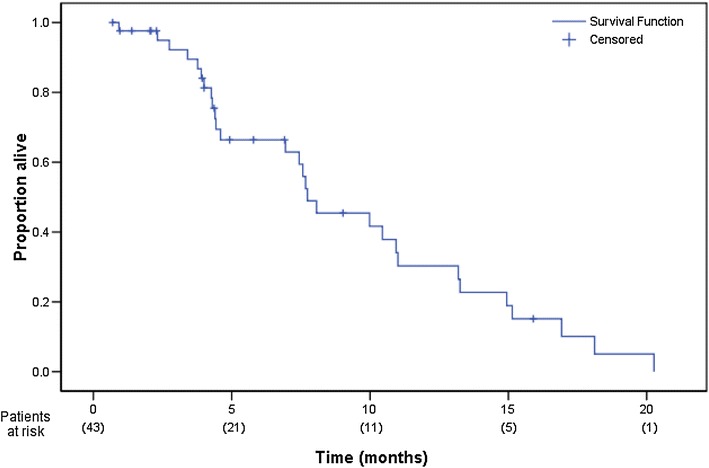
Overall survival
Discussion
The aim of this study was to determine the objective response rate of a combination of capecitabine and oxaliplatin in patients with advanced gall bladder or biliary tract cancer. The hypothesis was that a regimen with an objective response rate of >40 % would warrant further investigation, while a regimen with a response rate of <20 % would not be of further interest. Six patients discontinued chemotherapy with this regimen prior to the first planned disease re-assessment due to toxicity, which is higher than the patient withdrawal rate with this regimen in other tumour types, although the grade 3/4 toxicities were comparable to previous reports of this regimen in other tumour types. In total, 10 patients did not undergo repeat disease assessments for response. Ten of 33 patients assessable for response had an objective tumour response for an overall response rate of 30.3 %. However, the objective response rate, based on an intention to treat, in this study was 23 %, and so we do not recommend further study of this regimen as a first-line therapy regimen patients with advanced gall bladder or biliary tract cancer.
A similar response rate (21 %; 95 % CI 9–38 %) was observed in another study in which two 3-weekly cycles of capecitabine (1000 mg/m2 bid, days 1–14) plus oxaliplatin (130 mg/m2 i.v., on day1) were followed by XELOX-RT (radiotherapy [50.4 Gy] combined with capecitabine [750–675 mg/m2 bid] every radiotherapy day and oxaliplatin [40–30 mg/m2] once weekly [22]). The maximum tolerated doses of oxaliplatin and capecitabine when combined with radiotherapy were 30 and 675 mg/m2, respectively. Five patients became operable following this chemo-radiotherapy regimen, with three R0 resections and this corresponded to a two-year survival of 28 %, and with an estimated local tumour control rate at 2 years of 72 %. Objective response rates of between 18 and 41 % have been reported with most other oxaliplatin–containing regimens [23–27]. Similarly, response rates of 6.6–40.6 % have been seen with a number of other combination chemotherapy regimens in this disease (Table 3).
Table 3.
Chemotherapy studies in advanced biliary tract cancer
| Author (year) | Drug | Number of patients | Response rate (%) |
|---|---|---|---|
| Taal (1993) [32] | MMC | 30 | 10 |
| Patt (1996) [33] | 5FU | 32 | 34 |
| Interferon alfa-2b | |||
| Patt (2001) [34] | Cisplatin Interferon alpha-2b | 41 | 21 |
| Doxorubicin | |||
| 5 FU | |||
| Penz (2001) [35] | Gemcitabine | 32 | 22 |
| Murad (2003) [36] | Gemcitabine | 26 | 31 |
| 5FU | |||
| Kornek (2001) [37] | MMC/Capecitabine vs | 51 | 31 |
| MMC/gemcitabine | 20 | ||
| Ueno (2004) [38] | S1 | 19 | 21 |
| Alberts (2005) [39] | Gem | 42 | 9.5 |
| 5FU | |||
| Leucovorin | |||
| Furuse (2006) [40] | Uracil-tegafur | 24 | 12.5 |
| Doxorubicin | |||
| Kim (2006) [41] | Gemcitabine | 29 | 34.5 |
| Cisplatin | |||
| Okusaka (2006) [42] | Gemcitabine | 40 | 17.5 |
| Park (2006) [43] | Gemcitabine | 27 | 33.3 |
| Cisplatin | |||
| Hong (2007) [44] | Capecitabine | 32 | 40.6 |
| Cisplatin | |||
| Manzione (2007) [23] | Gemcitabine | 34 | 41 |
| Oxaliplatin | |||
| Furuse (2008) [45] | S1 | 40 | 35 |
| Kim (2008) [46] | S1 | 51 | 30 |
| Cisplatin | |||
| Oh (2008) [27] | S1 | 15 | 6.7 |
| Oxaliplatin | |||
| Furuse (2009) [47] | Uracil-tegafur | 61 | 6.6 |
| Doxorubicin | |||
| Kim (2009) [25] | Gemcitabine | 40 | 15 |
| Oxaliplatin | |||
| Sasaki (2009) [48] | S1 | 28 | 34.3 |
| Gemcitabine | |||
| Gruenberger (2010) [30] | Cetuximab | 30 | 63 |
| Gemcitabine | |||
| Oxaliplatin | |||
| Gunnlaugsson (2010) [22] | Capecitabine | 39 | 21 |
| Oxaliplatin | |||
| Kanai (2010) [49] | S1 | 25 | 30.4 |
| Gemcitabine | |||
| Karachaliou (2010) [24] | Irinotecan | 28 | 17.9 |
| Oxaliplatin | |||
| Chung (2011) [50] | Irinotecan | 39 | 20.5 |
| Gemcitabine |
A further study [28] without radiotherapy was very similar to ours and looked at oxaliplatin (130 mg/m2) and capecitabine (1000 mg/m2 bid, days 1–14) 3 weekly as firstline treatment in advanced biliary carcinoma. 65 patients were stratified prospectively into two groups based on location of the primary (gallbladder or extra hepatic cholangiocarcinoma (GBC/ECC) versus intrahepatic mass-forming type cholangiocarcinoma (ICC)). The response rates overall were significantly higher in the GBC/ECC (27 % OR, 49 % SD) than ICC group (no objective response, 33 % SD). Median survival was 12.8 months (CI 95 % 10–16.8 months) for for GBC or ECC compared with 5.2 months in the ICC (CI 95 % 12.7–20.5 months). The morphologically distinct presentations appear to differ in their presentations and response to cytotoxic drugs [29]. Our study replicates similar response rates but our survival times were shorter (between those of ICC and GBC/ECC groups) which may reflect many reasons, such as overall health of the included patients; differences in such characteristics will be more apparent given the small numbers.
A GERCOR study [30] has looked at the combination of gemcitabine (1000 mg/m2 day 1) and oxaliplatin (100 mg/m2 day 2) fortnightly as firstline treatment in biliary tract carcinoma and observed (again with small numbers—56 total) similar response rates to ours but also that the combination was well tolerated in frailer patients (it included patients of performance status (PS) >2 in one of the two groups). Group A (PS 0–2, bilirubin <2.5× ULN) showed OR in 36 % (95 % CI 18.7–52.3 %), stable disease 26 %, progressive disease 39 %. Median PFS was 5.7 months and OS was 15.7 months. Group B (PS >2 and/or bilirubin >2.5× ULN) showed OR 22 % (95 % CI 6.5–37.4 %), stable disease 30 %, progressive disease 48 %. PFS was 3.9 months and OS 7.6 months. A two weekly regimen would mean slightly more hospital visits when compared with oxaliplatin and capecitabine but again may provide an alternative regimen to the current gold standard.
Following completion of our study, the combination of cisplatin (25 mg/m2) followed by gemcitabine (1000 mg/m2 days 1 and 8) every 3 weeks for eight cycles is now established as the standard of care in this disease following a phase III randomised trial in comparison with gemcitabine monotherapy. This regimen results in a disease control rate of 81.4 %, median progression-free survival of 8.0 months, and a median overall survival of 11.7 months [31]. The results of our study suggest that the combination of capecitabine with oxaliplatin has lower disease control and shorter overall survival than cisplatin in combination with gemcitabine. However, the combination of capecitabine and oxaliplatin remains a regimen with activity in this disease, and can be considered as an alternative treatment option for patients in whom cisplatin and/or gemcitabine are contra-indicated.
Authors’ contributions
Patient recruitment and collection of clinical data: FC, LW, ME, TM, MH, PBZ, TRJE; design of study: FC, LW, ME, TM, MH, SH, JP, TRJE; data analysis: JG, JP, SH, TRJE; final manuscript draft: JG, KB, JP, TRJE. All authors read and approved final manuscript.
Acknowledgements
The authors are very grateful to the nursing staff and staff in the Clinical Trials Unit who made this study possible.
Competing interests
Funding support as noted. Professor Jeff Evans has received honoraria and consultancies from Roche for capecitabine, in addition to speaker’s fees for a presentation.
Funding support
The study was coordinated by the Cancer Research UK Glasgow Clinical Trials Unit which is supported by a programme grant from Cancer Research UK. Capecitabine was supplied by Roche Products (UK), and oxaliplatin was supplied by Sanofi-Aventis (UK).
Contributor Information
J. S. Graham, Email: Janet.graham@ggc.scot.nhs.uk
K. Boyd, Phone: +44 141 301 7147, Email: boyka01@gmail.com
F. Y. Coxon, Email: Fareeda.Coxon@nuth.nhs.uk
L. R. Wall, Email: Lucy.Wall@nhslothian.scot.nhs.uk
M. M. Eatock, Email: martin.eatock@belfasttrust.hscni.net
T. S. Maughan, Email: tim.maughan@oncology.ox.ac.uk
M. Highley, Email: martin.highley@nhs.net
E. Soulis, Email: Eileen.Soulis@glasgow.ac.uk
S. Harden, Email: Sharon.Tuck@ed.ac.uk
P. Bützberger-Zimmerli, Email: Priska.Buetzberger@ksb.ch
T. R. J. Evans, Email: j.evans@beatson.gla.ac.uk
References
- 1.Hejna M, Pruckmayer M, Raderer M. The role of chemotherapy and radiation in the management of biliary cancer: a review of the literature. Eur J Cancer. 1998;34(7):977–986. doi: 10.1016/S0959-8049(97)10166-6. [DOI] [PubMed] [Google Scholar]
- 2.Glimelius B, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996;7(6):593–600. doi: 10.1093/oxfordjournals.annonc.a010676. [DOI] [PubMed] [Google Scholar]
- 3.Davis HL, Jr, Ramirez G, Ansfield FJ. Adenocarcinomas of stomach, pancreas, liver, and biliary tracts. Survival of 328 patients treated with fluoropyrimidine therapy. Cancer. 1974;33(1):193–197. doi: 10.1002/1097-0142(197401)33:1<193::AID-CNCR2820330128>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 4.Falkson G, MacIntyre JM, Moertel CG. Eastern cooperative Oncology Group experience with chemotherapy for inoperable gallbladder and bile duct cancer. Cancer. 1984;54(6):965–969. doi: 10.1002/1097-0142(19840915)54:6<965::AID-CNCR2820540603>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 5.Haskell CM. Cancer of the liver. In: Haskell CM, editor. Cancer Treatment. Philadelphia: WB Saunders; 1980.
- 6.Kato K, et al. Efficacy of 48-hour infusion of 5-fluorouracil for gall bladder cancer. Gan To Kagaku Ryoho. 1993;20(15):2341–2344. [PubMed] [Google Scholar]
- 7.Takada T, et al. Comparison of 5-fluorouracil, doxorubicin and mitomycin C with 5-fluorouracil alone in the treatment of pancreatic-biliary carcinomas. Oncology. 1994;51(5):396–400. doi: 10.1159/000227373. [DOI] [PubMed] [Google Scholar]
- 8.Schuller J, et al. Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients. Cancer Chemother Pharmacol. 2000;45(4):291–297. doi: 10.1007/s002800050043. [DOI] [PubMed] [Google Scholar]
- 9.Cassidy J, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26(12):2006–2012. doi: 10.1200/JCO.2007.14.9898. [DOI] [PubMed] [Google Scholar]
- 10.Cassidy J, et al. A Phase I study of capecitabine in combination with oral leucovorin in patients with intractable solid tumors. Clin Cancer Res. 1998;4(11):2755–2761. [PubMed] [Google Scholar]
- 11.Comella P, et al. Capecitabine plus oxaliplatin for the first-line treatment of elderly patients with metastatic colorectal carcinoma: final results of the Southern Italy Cooperative Oncology Group Trial 0108. Cancer. 2005;104(2):282–289. doi: 10.1002/cncr.21167. [DOI] [PubMed] [Google Scholar]
- 12.Diaz-Rubio E, et al. Capecitabine (Xeloda) in combination with oxaliplatin: a phase I, dose-escalation study in patients with advanced or metastatic solid tumors. Ann Oncol. 2002;13(4):558–565. doi: 10.1093/annonc/mdf065. [DOI] [PubMed] [Google Scholar]
- 13.Ducreux M, et al. Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/leucovorin plus oxaliplatin (FOLFOX-6) as first-line treatment for metastatic colorectal cancer. Int J Cancer. 2010;128(3):682–690. doi: 10.1002/ijc.25369. [DOI] [PubMed] [Google Scholar]
- 14.Haller DG, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29(11):1465–1471. doi: 10.1200/JCO.2010.33.6297. [DOI] [PubMed] [Google Scholar]
- 15.Mackean M, et al. Phase I and pharmacologic study of intermittent twice-daily oral therapy with capecitabine in patients with advanced and/or metastatic cancer. J Clin Oncol. 1998;16(9):2977–2985. doi: 10.1200/JCO.1998.16.9.2977. [DOI] [PubMed] [Google Scholar]
- 16.Blum JL, et al. Multicenter, Phase II study of capecitabine in taxane-pretreated metastatic breast carcinoma patients. Cancer. 2001;92(7):1759–1768. doi: 10.1002/1097-0142(20011001)92:7<1759::AID-CNCR1691>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 17.Oshaughnessy JA, et al. Randomized, open-label, phase II trial of oral capecitabine (Xeloda) vs a reference arm of intravenous CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Ann Oncol. 2001;12(9):1247–1254. doi: 10.1023/A:1012281104865. [DOI] [PubMed] [Google Scholar]
- 18.Okines AF, et al. Meta-analysis of the REAL-2 and ML17032 trials: evaluating capecitabine-based combination chemotherapy and infused 5-fluorouracil-based combination chemotherapy for the treatment of advanced oesophago-gastric cancer. Ann Oncol. 2009;20(9):1529–1534. doi: 10.1093/annonc/mdp047. [DOI] [PubMed] [Google Scholar]
- 19.Mathe G, et al. Oxalato-platinum or 1-OHP, a third-generation platinum complex: an experimental and clinical appraisal and preliminary comparison with cis-platinum and carboplatinum. Biomed Pharmacother. 1989;43(4):237–250. doi: 10.1016/0753-3322(89)90003-6. [DOI] [PubMed] [Google Scholar]
- 20.Diaz-Rubio E, et al. Phase III study of capecitabine plus oxaliplatin compared with continuous-infusion fluorouracil plus oxaliplatin as first-line therapy in metastatic colorectal cancer: final report of the Spanish Cooperative Group for the Treatment of Digestive Tumors Trial. J Clin Oncol. 2007;25(27):4224–4230. doi: 10.1200/JCO.2006.09.8467. [DOI] [PubMed] [Google Scholar]
- 21.Therasse P, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.Gunnlaugsson A, et al. Multicentre phase I-II trial of capecitabine and oxaliplatin in combination with radiotherapy for unresectable pancreatic and biliary tract cancer: the CORGI-U study. Radiother Oncol. 2010;95(3):292–297. doi: 10.1016/j.radonc.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Manzione L, Romano R, Germano D. Chemotherapy with gemcitabine and oxaliplatin in patients with advanced biliary tract cancer: a single-institution experience. Oncology. 2007;73(5–6):311–315. doi: 10.1159/000134239. [DOI] [PubMed] [Google Scholar]
- 24.Karachaliou N, et al. A multicenter phase II trial with irinotecan plus oxaliplatin as first-line treatment for inoperable/metastatic cancer of the biliary tract. Oncology. 2010;78(5–6):356–360. doi: 10.1159/000320462. [DOI] [PubMed] [Google Scholar]
- 25.Kim HJ, et al. A phase II study of gemcitabine in combination with oxaliplatin as first-line chemotherapy in patients with inoperable biliary tract cancer. Cancer Chemother Pharmacol. 2009;64(2):371–377. doi: 10.1007/s00280-008-0883-7. [DOI] [PubMed] [Google Scholar]
- 26.Kim KP, et al. Phase II study of S-1 combined with oxaliplatin as therapy for patients with metastatic biliary tract cancer: influence of the CYP2A6 polymorphism on pharmacokinetics and clinical activity. Br J Cancer. 2011;104(4):605–612. doi: 10.1038/bjc.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh SY, et al. Phase II trial of S-1 in combination with oxaliplatin in previously untreated patients with recurrent or inoperable biliary tract cancer. Chemotherapy. 2008;54(6):479–484. doi: 10.1159/000159624. [DOI] [PubMed] [Google Scholar]
- 28.Nehls O, et al. Capecitabine plus oxaliplatin as first-line treatment in patients with advanced biliary system adenocarcinoma: a prospective multi centre phase II trial. Br J Cancer. 2008;98:309–313. doi: 10.1038/sj.bjc.6604178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarnagin WR, et al. Differential cell-cycle-regulatory protein expression in biliary tract adenocarcinoma; correlation with anatomic site, pathologic variables, and clinical outcome. J Clin Oncol. 2006;24:1151–1160. doi: 10.1200/JCO.2005.04.6631. [DOI] [PubMed] [Google Scholar]
- 30.Andre T, et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol. 2004;15(9):1339–1343. doi: 10.1093/annonc/mdh351. [DOI] [PubMed] [Google Scholar]
- 31.Valle J, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 32.Taal BG, et al. Phase II trial of mitomycin C (MMC) in advanced gallbladder and biliary tree carcinoma. An EORTC Gastrointestinal Tract Cancer Cooperative Group Study. Ann Oncol. 1993;4(7):607–609. doi: 10.1093/oxfordjournals.annonc.a058597. [DOI] [PubMed] [Google Scholar]
- 33.Patt YZ, et al. Phase II trial of intravenous flourouracil and subcutaneous interferon alfa-2b for biliary tract cancer. J Clin Oncol. 1996;14(8):2311–2315. doi: 10.1200/JCO.1996.14.8.2311. [DOI] [PubMed] [Google Scholar]
- 34.Patt YZ, et al. Phase II trial of cisplatin, interferon alpha-2b, doxorubicin, and 5-fluorouracil for biliary tract cancer. Clin Cancer Res. 2001;7(11):3375–3380. [PubMed] [Google Scholar]
- 35.Penz M, et al. Phase II trial of two-weekly gemcitabine in patients with advanced biliary tract cancer. Ann Oncol. 2001;12(2):183–186. doi: 10.1023/A:1008352123009. [DOI] [PubMed] [Google Scholar]
- 36.Murad AM, et al. Phase II trial of the use of gemcitabine and 5-fluorouracil in the treatment of advanced pancreatic and biliary tract cancer. Am J Clin Oncol. 2003;26(2):151–154. doi: 10.1097/00000421-200304000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Kornek GV, et al. Mitomycin C in combination with capecitabine or biweekly high-dose gemcitabine in patients with advanced biliary tract cancer: a randomised phase II trial. Ann Oncol. 2004;15(3):478–483. doi: 10.1093/annonc/mdh096. [DOI] [PubMed] [Google Scholar]
- 38.Ueno H, et al. Phase II study of S-1 in patients with advanced biliary tract cancer. Br J Cancer. 2004;91(10):1769–1774. doi: 10.1038/sj.bjc.6602208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alberts SR, et al. Gemcitabine, 5-fluorouracil, and leucovorin in advanced biliary tract and gallbladder carcinoma: a North Central Cancer Treatment Group phase II trial. Cancer. 2005;103(1):111–118. doi: 10.1002/cncr.20753. [DOI] [PubMed] [Google Scholar]
- 40.Furuse J, et al. Early phase II study of uracil-tegafur plus doxorubicin in patients with unresectable advanced biliary tract cancer. Jpn J Clin Oncol. 2006;36(9):552–556. doi: 10.1093/jjco/hyl075. [DOI] [PubMed] [Google Scholar]
- 41.Kim ST, et al. A Phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. Cancer. 2006;106(6):1339–1346. doi: 10.1002/cncr.21741. [DOI] [PubMed] [Google Scholar]
- 42.Okusaka T, et al. Phase II study of single-agent gemcitabine in patients with advanced biliary tract cancer. Cancer Chemother Pharmacol. 2006;57(5):647–653. doi: 10.1007/s00280-005-0095-3. [DOI] [PubMed] [Google Scholar]
- 43.Park BK, et al. Phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. J Gastroenterol Hepatol. 2006;21(6):999–1003. doi: 10.1111/j.1440-1746.2006.04230.x. [DOI] [PubMed] [Google Scholar]
- 44.Hong YS, et al. Phase II study of capecitabine and cisplatin in previously untreated advanced biliary tract cancer. Cancer Chemother Pharmacol. 2007;60(3):321–328. doi: 10.1007/s00280-006-0380-9. [DOI] [PubMed] [Google Scholar]
- 45.Furuse J, et al. S-1 monotherapy as first-line treatment in patients with advanced biliary tract cancer: a multicenter phase II study. Cancer Chemother Pharmacol. 2008;62(5):849–855. doi: 10.1007/s00280-007-0673-7. [DOI] [PubMed] [Google Scholar]
- 46.Kim YJ, et al. A phase II trial of S-1 and cisplatin in patients with metastatic or relapsed biliary tract cancer. Ann Oncol. 2008;19(1):99–103. doi: 10.1093/annonc/mdm439. [DOI] [PubMed] [Google Scholar]
- 47.Furuse J, et al. A phase II study of uracil-tegafur plus doxorubicin and prognostic factors in patients with unresectable biliary tract cancer. Cancer Chemother Pharmacol. 2009;65(1):113–120. doi: 10.1007/s00280-009-1011-z. [DOI] [PubMed] [Google Scholar]
- 48.Sasaki T, et al. Multicenter, phase II study of gemcitabine and S-1 combination chemotherapy in patients with advanced biliary tract cancer. Cancer Chemother Pharmacol. 2009;65(6):1101–1107. doi: 10.1007/s00280-009-1115-5. [DOI] [PubMed] [Google Scholar]
- 49.Kanai M, et al. A multi-institution phase II study of gemcitabine/S-1 combination chemotherapy for patients with advanced biliary tract cancer. Cancer Chemother Pharmacol. 2010;67(6):1429–1434. doi: 10.1007/s00280-010-1443-5. [DOI] [PubMed] [Google Scholar]
- 50.Chung MJ, et al. Prospective phase II trial of gemcitabine in combination with irinotecan as first-line chemotherapy in patients with advanced biliary tract cancer. Chemotherapy. 2011;57(3):236–243. doi: 10.1159/000328021. [DOI] [PubMed] [Google Scholar]


