Fig. 4.
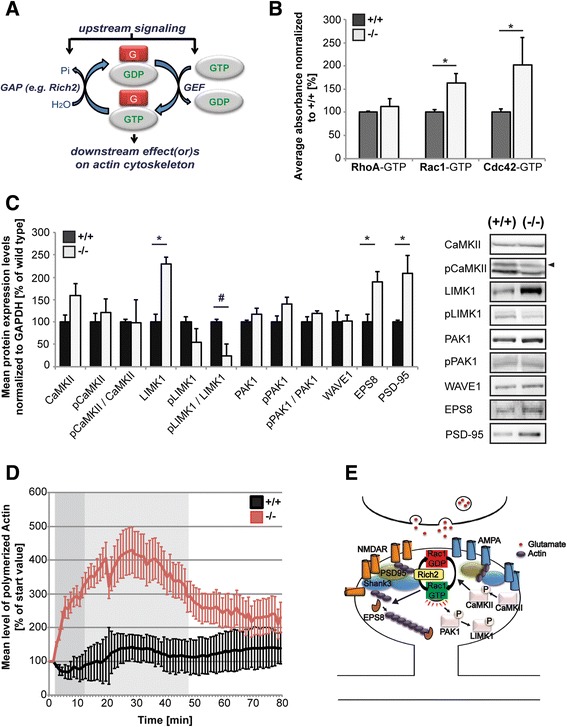
Functional analysis of impaired synaptic signaling caused by deletion of RICH2. a Schematic illustration of small G-protein GTPase-cycle. (G = G-protein (e.g. RHO, RAC1, CDC42), GAP = GTPase activating protein, GEF = guanine-nucleotide exchange factor, Pi = phosphate). G proteins are activated via GEF (guanine nucleotide exchange factor) upon replacement of GDP by GTP and inactivated via GAP (GTPase activating protein) due to hydrolysis of GTP via GAPs. b Using G-LISA activation assays, RHOA, RAC1, and CDC42 activity was measured in P2 fractions from hippocampal tissue lysates of three animals per group in technical triplicates. The results show a significantly increased activation (GTP-binding) of RAC1 and CDC42 in RICH2−/− mice compared to wild type controls (unpaired t-test, Rac1: df = 4, p = 0.0476, t = 2.8243; Cdc42: df = 4, p = 0.0275, t = 3.3908). No significant difference was detected for RhoA (unpaired t-test, df = 4, p = 0.4727, t = 0.7919). c Western blot analysis (showing relative percentages of mean + SEM) of hippocampal synapse-enriched P2-fractions extracted from P70 wild type (+/+, n = 3), and knock-out (−/−, n = 3) mice. Proteins were normalized to GAPDH expression levels. A significant increase in LIMK1 expression levels can be observed in RICH2−/− animals (unpaired t-test, LIMK: df = 4, p = 0.005, t = 5.6029). Given that the levels of phosphorylated LIMK1 (pLIMK1) remain unchanged, a difference in the ratio of pLIMK1 / LIMK1 can be observed, however, only as trend (pLIMK/LIMK: df = 4, p = 0.0545, t = 2.6923). In addition, the levels of EPS8 and PSD-95 are significantly increased in RICH2−/− mice (unpaired t-test, EPS8: df = 4, p = 0.0384, t = 3.039; PSD-95: df = 4, p = 0.0529, t = 2.7223). Right panel: Representative illustration from hippocampal P2-immunoblots. For each protein analyzed two representative immunonblot-signals are illustrated per genotype. d) Hippocampal P2 lysates from RICH2+/+ and RICH2−/− animals (n = 3) were used in an actin polymerization assay. The lysate was added to a solution with pyrene-conjugated actin and the increase in fluorescence intensity that occurs when pyrene G-actin (monomer) forms pyrene F-actin measured over a time-course of 80 min. P2 lysate from RICH2−/− mice is able to induce actin polymerization to a significantly higher amount within the first 30 min of the experiment compared to lysate from RICH2+/+ mice (repeated measure ANOVA, effect of genotype by time F1.14 = 5.402, p < 0.0001; effect of time F1.14 = 6.397, p < 0.0001; effect of the genotype F1.14 = 9.015, p = 0.040). e) Schematic overview of RAC1 downstream signaling pathways. In RICH2−/− mice, effects on spine morphology and actin polymerization most likely are mediated by activation of EPS8
