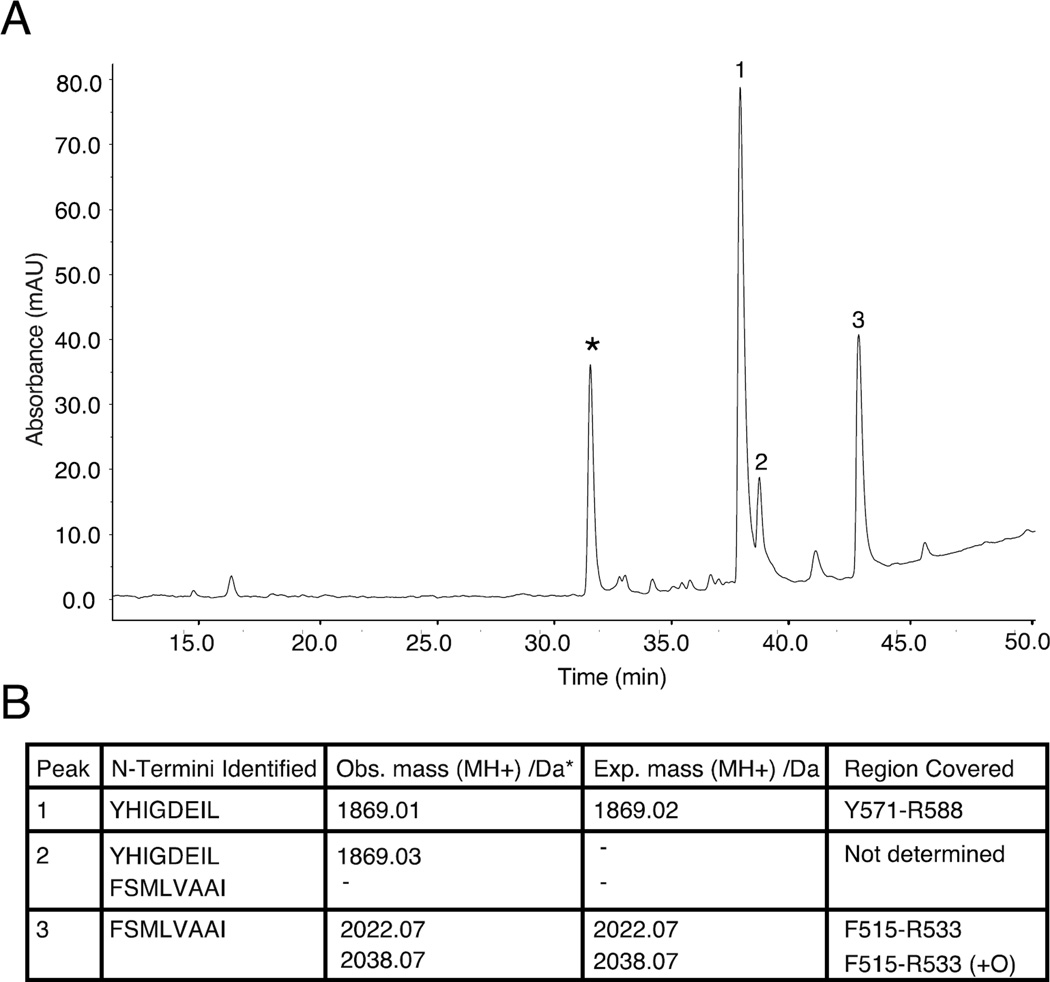
Figure 5.
Two tryptic peptides of A546T FAS1-4 are highly insoluble. Fibrillation of tryptic peptides of A546T FAS1-4 reveals two aggregation prone peptides in the pellet fraction. A, After extensive trypsin degradation and subsequent fibrillation, the tryptic peptides from A546T FAS1-4 were separated into a pellet fraction and a supernatant fraction. The aggregated peptides in the pellet fraction were separated using RP-HPLC, and four major peaks were observed. The peak marked with an asterisk did not contain any peptide detected by N-terminal sequencing or mass spectrometry. B, Table of identified N-termini and observed masses from the three peaks in panel A and with expected masses and regions covered in A546T FAS1-4.