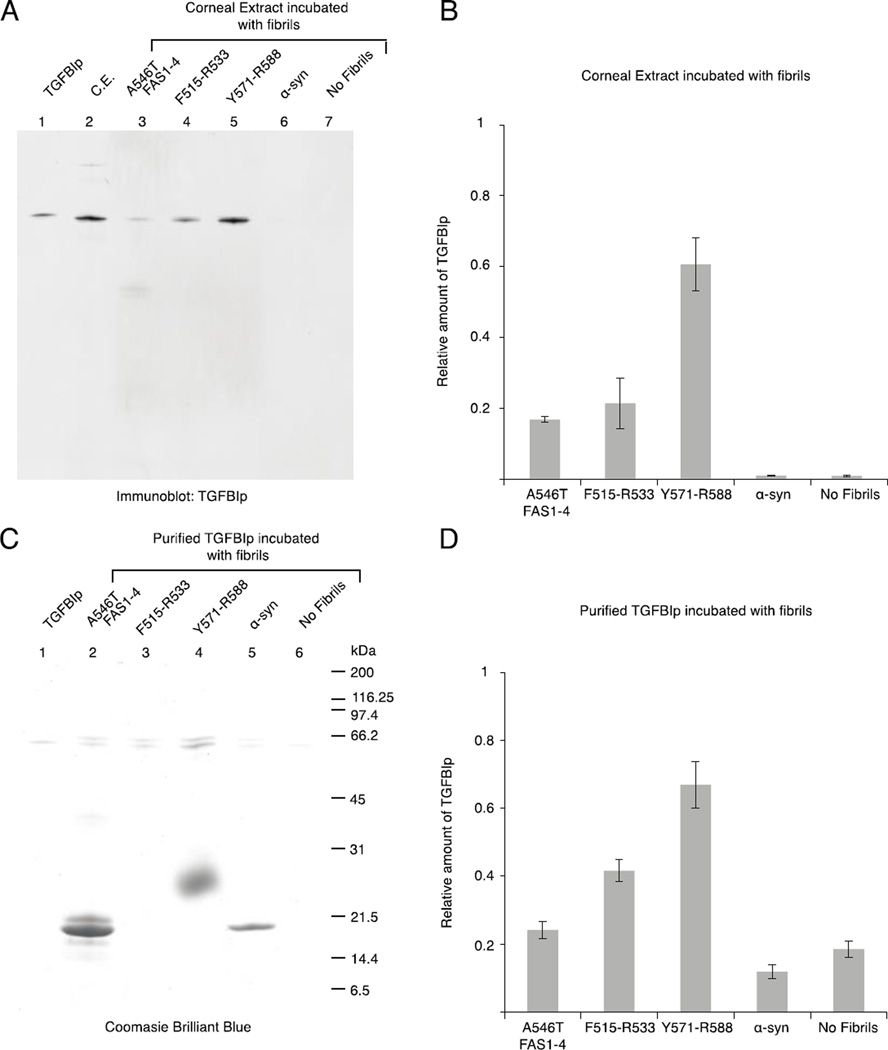
Figure 8.
WT TGFBIp aggregation is induced by fibril seeds of the core peptides. Corneal extract (A) or recombinant TGFBIp (C) were incubated with fibrillated samples of A546T FAS1-4, F515-R533, Y571-R588, α-synuclein, or no fibrils. Subsequently, the fibrils and interaction partners were extensively washed, and the proteins were resolved using SDS-PAGE. A, The proteins from the corneal extract were visualized by immunoblotting using anti-TGFBIp polyclonal antiserum. Purified TGFBIp and corneal extract (C.E.) were included as controls. B, Relative amount of TGFBIp from the corneal extract bound to the fibrils, n = 3, error bars = standard deviation. C, Purified recombinant TGFBIp was visualized using Coomassie brilliant blue staining. Recombinant TGFBIp was included as a control. F515-R533 migrated with the dye front, and therefore, it is not visible on the gel (Lane 3); Y571-R588 migrated above its size as a very fuzzy band (Lane 4). D, Relative amount of purified TGFBIp bound to the fibrils, n = 3, error bars represent the standard deviation. Both corneal TGFBIp and recombinant TGFBIp aggregate in the presence of fibrillated core peptides, with Y571-R588 causing the highest degree of aggregation.