Fig. 2.
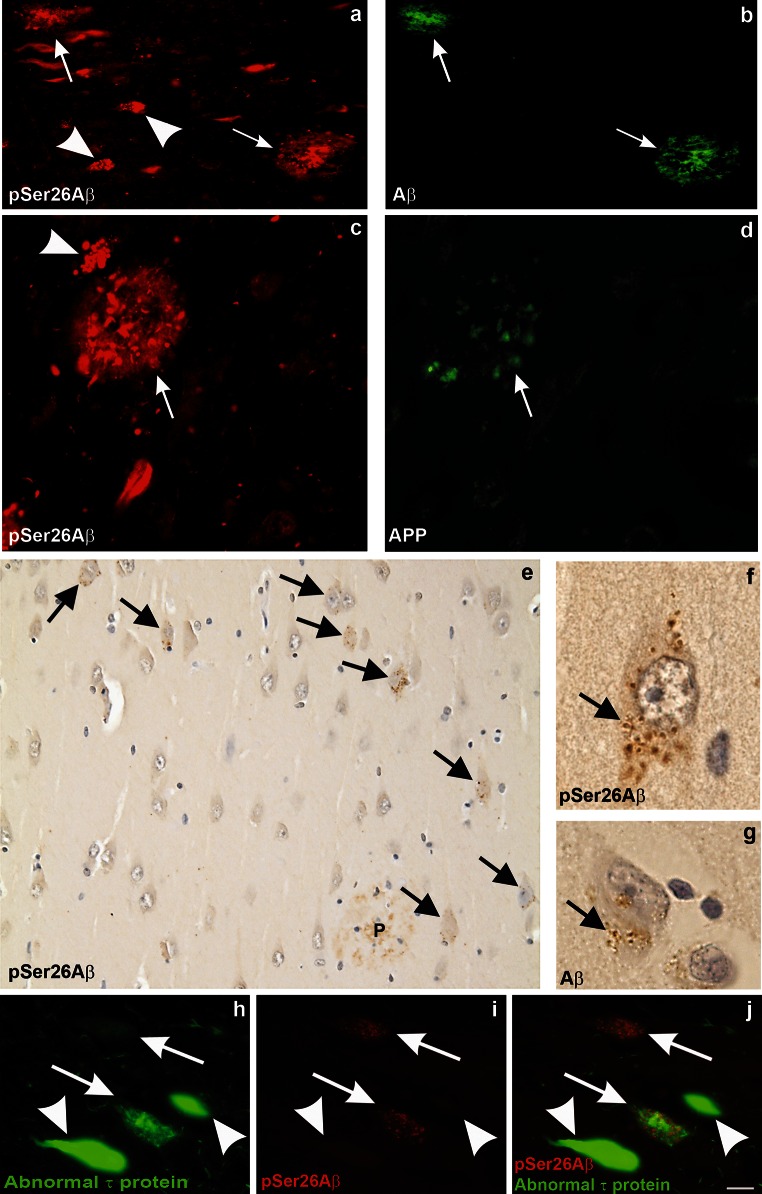
Detection of intraneuronal pSer26Aβ aggregates and GVDs in human AD brains. Detection of pSer26Aβ in extracellular (arrows) and intraneuronal (arrowheads) deposits in the hippocampal CA1 subfield (a, c, e, f, g, i). The extracellular Aβ plaques are co-stained with anti-Aβ17–24 (4G8) (b), and anti-APP antibody (22C11) (d).The central amyloid core is stained with SA6192 and 4G8 but not with anti-APP indicating the co-deposition of pSer26Aβ together with non-phosphorylated Aβ in plaques (Supplementary Fig. 5a–c). Note the intraneuronal globular aggregates reactivity is selectively observed with pSer26Aβ (arrowhead in a, c), but not with APP antibodies. Immunohistochemical analysis demonstrates strong intraneuronal granular cytoplasmic pSer26Aβ inclusions (arrows in e), and only weakly stained extracellular pSer26Aβ-positive plaques (P in e) (Supplementary Fig. 4d). These granular inclusions exhibit the morphological pattern of granulovacuolar degeneration (GVD) and most frequently occur in the CA1-subiculum area of the hippocampal formation (arrow in f). GVD was also detected by anti-Aβ17–24 staining (arrow in g). pSer26Aβ-positive GVD lesions colocalized with abnormal-phosphorylated τ in neurons (arrows in h–j). Note that neurofibrillary tangles were not labelled with anti-pSer26Aβ antibody (arrowhead in h–j). The panels in this figure are representative images from 4 different AD brains (a, b case # 7; c, d case # 3, e–g case # 1 and h, i case # 5 of supplementary Table 2). Scale bars a and b 50 µm; c and d 30 µm; e 20 µm; f and g 5 µm; h–j 20 µm