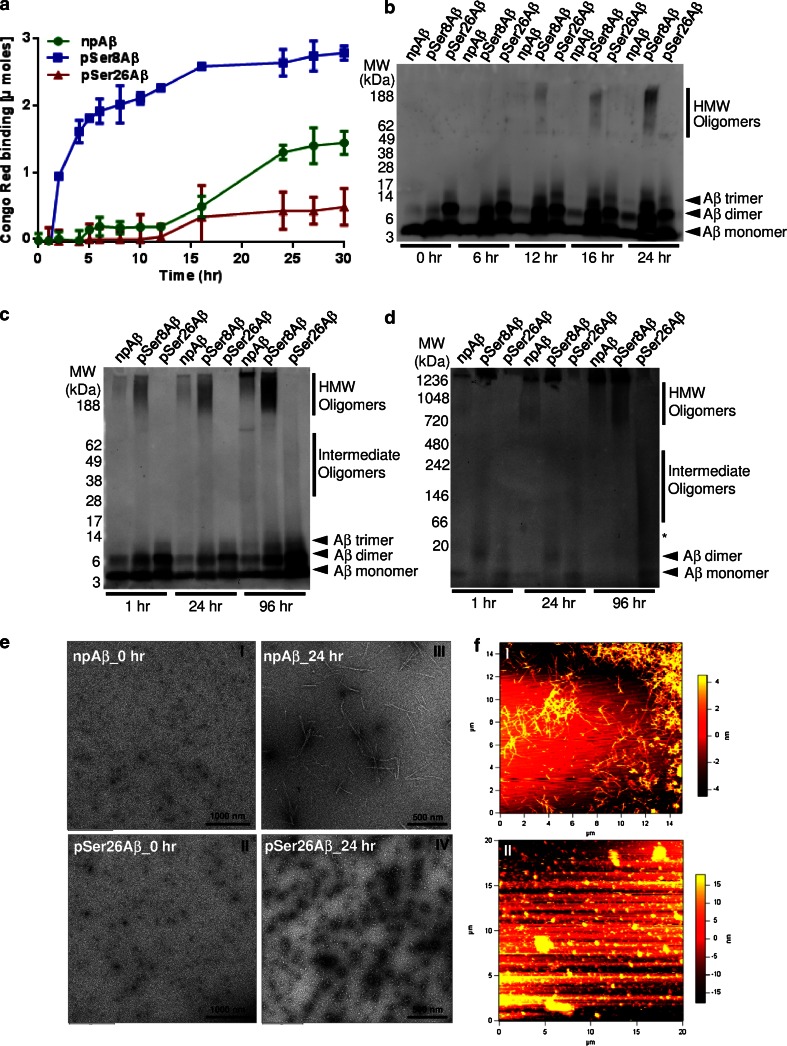
Fig. 3.
pSer26Aβ selectively forms oligomers without fibril formation. a Congo Red (CR) binding assay showing the decreased CR dye binding to pSer26Aβ as compared to npAβ and pSer8Aβ peptides. b SDS-PAGE and Western immunoblot detection of Aβ variants after different times of aggregation (see also supplementary Fig. 7). SDS-PAGE (c) and native-PAGE (d) analysis of the aggregates collected at different incubation time demonstrates the lack of HMW pSer26Aβ assemblies, even after prolonged incubation time (96 h). Monoclonal 82E1 antibody was used for immunoblotting. e Transmission electron microscopy (TEM) images demonstrate granular non-aggregated structures of npAβ and pSer26Aβ peptide samples at 0 h (e I, II). After 24 h of incubation, mature fibrils are only seen with npAβ (e III), whereas pSer26Aβ predominantly shows spherical non-fibrillar chain-like globular structures (e IV). f Atomic force microscopy (AFM) images of npAβ and pSer26Aβ after 24 h of aggregation further confirm the formation of fibrillar aggregates of npAβ (f, i) and non-fibrillar globular assemblies of pSer26Aβ peptide (f II, see also Supplementary Fig. 8)