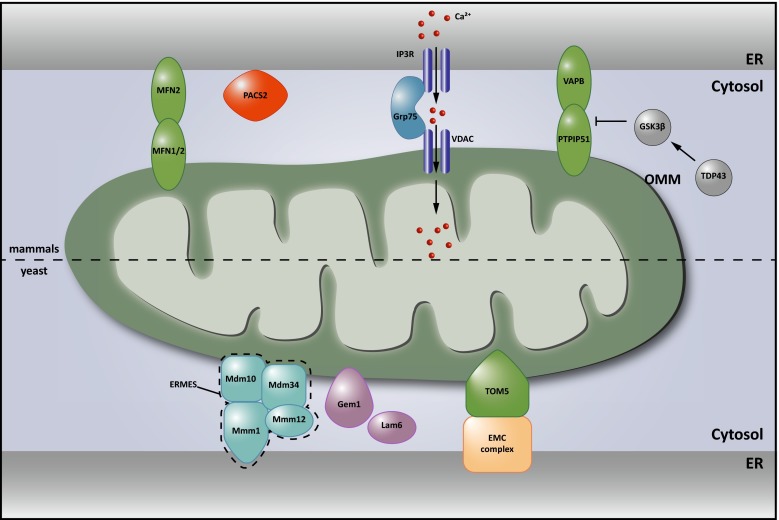
Fig. 2.
Structural components of ER-mitochondria contact sites. Upper part in mammalian cells, dimers between endoplasmic reticulum (ER)-localized Mitofusin (MFN) 2 and mitochondrial MFN1/2 were the first proposed protein tethers. Charcot–Marie–Tooth (CMT)-causing mutations in MFN2 are believed to decrease ER-mitochondrial contact, contributing to the disease. The interaction of VAMP-associated protein B and C (VAPB) in the ER membrane with protein tyrosine phosphatase-interacting protein 51 (PTPIP51) in the outer mitochondrial membrane (OMM) also contributes to anchoring the mitochondria-associated membrane (MAM) to the mitochondrial membrane. This interaction is inhibited by TAR DNA-binding protein 43 (TDP43) in a glycogen synthase kinase 3β (GSK3β) dependent manner. Both these proteins are implied in neurodegeneration (see Table 1; Boxes 1, 2). The amyotrophic lateral sclerosis (ALS)-causing P56S mutation in VAPB on the other hand increases the physical interaction between the ER and mitochondria (Table 1; Box 2). Phosphofurin acidic cluster protein 2 (PACS2) is established as an essential component of these contacts. The levels of PACS2 are found to be altered in the brains of Alzheimer’s dementia (AD) patients (see Table 1; and Box 1). A functional rather than a structural component of the MAMs is formed by a complex between ER-resident inositol 1,4,5-triphosphate (IP3) channels and the mitochondria resident voltage-dependent anion channel (VDAC), which are bridged by Grp75 and important for calcium shuttling between ER and mitochondria (see Fig. 3). Lower part in Saccharomyces cerevisiae, two tethering complexes are known: the endoplasmic reticulum (ER) mitochondria encounter structure (ERMES), composed of the mitochondrial Mdm10 and Mdm34 proteins, the ER-based Mmm1 protein and the cytosolic Mdm12 protein was the first tether to be described. A second tether important for yeast phospholipid metabolism is achieved through the interaction between the ER membrane complex (EMC) and the outer mitochondrial membrane translocate complex 5 (TOM5). The Miro GTPase Gem1 is a regulatory subunit of ERMES and also Lam6 plays a modulating role determining the extent of membrane contact. It is currently not known whether mammalian orthologs of these components play similar roles in mammalian MAMs