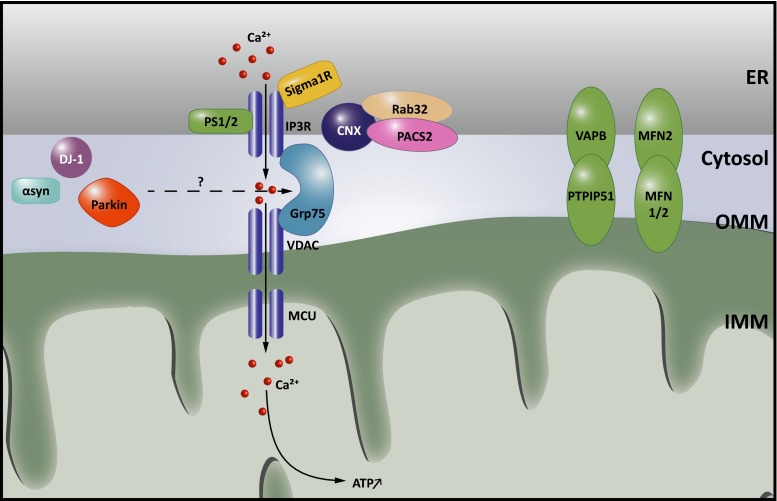
Fig. 4.
Calcium signaling at ER-mitochondria membrane contact sites. Transfer of calcium to mitochondria requires calcium hotspots, which can be achieved at mitochondria-associated membranes (MAMs) owing to the quasi-synaptic structure of this membrane contact site (MCS). Shuttling of calcium is needed during a variety of responses, as it can both stimulate ATP synthesis and promote mitophagy and apoptosis. Efficient import of calcium at MAMs is mediated by Grp75, which brings the openings of the inositol 1,4,5-triphosphate receptor (IP3R) calcium channels in the endoplasmic reticulum (ER) in close vicinity to the voltage dependent anion channel (VDAC) in the outer mitochondrial membrane (OMM). Opening of the IP3Rs is regulated by a set of proteins present at or recruited to MAMs, including calnexin (CNX), the Sigma non-opioid intracellular receptor 1 (Sigma1R), presenilin 1 and 2 (PS1 and PS2). The presence of CNX at MAMs in turn is regulated by the sorting proteins Rab32 and phosphofurin acidic cluster protein 2 (PACS2), as well as by palmitoylation of CNX. The association of Sigma1R with MAMs on the other hand depends on cholesterol levels. Dysregulation of calcium signaling at MAMs is strongly implied in neurodegenerative disorders. PS1 and PS2 play a complex role regulating both the extent of ER-mitochondrial contact and calcium signaling. The Parkinson’s disease (PD)-associated Parkin, DJ1 and α-synuclein (α-Syn) were also found to affect calcium transfer at these contacts. Finally, defects in mitofusin 2 (MFN2) or VAMP-associated protein B and C (VAPB), which contribute to the structural integrity of the MCSs, affect the calcium crosstalk between the ER and mitochondria