| Patent Application Title: |
Heterocyclyl-Butanamide Derivatives |
| Patent Application Number: |
WO 2015/169421 A1 |
Publication date: |
12 November 2015 |
| Priority Application: |
EP 14001613.0 |
Priority date: |
7 May 2014 |
| Inventors: |
Buchstaller, H.-P.; Dorsch, D. |
| Assignee Company: |
Merck Patent GMBH; Frankfurter Strasse 250, 64293 Darmstadt (DE) |
| Disease Area: |
Cancer, multiple sclerosis, cardiovascular diseases, CNS injury, and different forms of inflammation |
Biological Target: |
Tankyrases (TANKs) and poly(ADP-ribose)polymerase-1 (PARP-1) |
| Summary: |
The invention in this patent application relates to 1-butanoylpiperidine derivatives represented generally by formula (I). These compounds inhibit the activity of tankyrases (TNKS) and poly(ADP-ribose)polymerase-1 (PARP-1) and may provide useful treatment for diseases such as cancer, multiple sclerosis, cardiovascular diseases, central nervous system injury, and different forms of inflammation. |
| The poly(ADP-ribose) polymerase (PARP) protein superfamily performs several cellular processes such as DNA repair, Wnt-signaling, mitotic apparatus formation, and cell death. Members of the family mediate the post-translational poly(ADP-ribosyl)ation modification of targeted proteins employing the catalytic function of nicotinamide adenine dinucleotide (NAD). They also mediate the polymerization of ADP-riboses via glycosidic bonds, creating long and branched ADP-ribose polymers. These polymers are thought to modify protein functions. Some of the members of this growing family of enzymes are named poly(ADP-ribose) polymerases (PARPs) such as PARP-1, PARP-2, PARP-3, and Vault-PARP. Other members of the family are named tankyrases (TNKS), such as TNKS-1 and TNKS-2. In humans, the PARP family members are encoded by 17 different genes. The first identified and the most studied member of the family is the nuclear enzyme PARP-1. For some time, it remained as the only known enzyme with poly(ADP-ribosyl)ation activity until studies showed that PARP-1 knock out mice can still perform poly(ADP-ribosyl)ation functions, suggesting the existence of other similar enzymes. Following these studies, researchers identified several other family members, although most of them are less studied. |
| PARP-1 contains three major functional domains: an amino terminal DNA-binding domain (DBD), a central automodification domain (AMD), and a carboxy terminal catalytic domain (CD). Studies have shown that PARP-1 is overexpressed in a variety of cancers and has been linked to prognosis of cancers, most notably breast cancer. A variety of endogenous and exogenous stress signals including those generated by oxidative, genotoxic, thermal, oncogenic, metabolic, and inflammatory stresses can trigger responses from PARP-1 that may in turn trigger pathological conditions such as cancer, inflammation related diseases, autoimmune diseases, neurodegenerative diseases, and metabolic stresses. Thus, the inhibition of PARP-1 activities may be beneficial as a therapeutic target to treat these diseases. |
| Tankyrases contain ankyrin-repeat protein–interaction domain, a sterile α-motif oligomerization domain (SAM), and a PARP catalytic domain (PCD), which differentiates them from other members of the PARP family. TNKS-1 and TNKS-2 (a.k.a. PARP-5A and PARP-5B) perform several cellular functions, including telomere homeostasis, mitotic spindle formation, vesicle transport linked to glucose metabolism, Wnt-β-catenin signaling, and viral replication. TNKS-1 activity appears to be essential for the polymerization of mitotic spindle-associated poly(ADP-ribose) and the accurate formation and maintenance of spindle bipolarity. Furthermore, its activity is required for normal telomere separation before anaphase. Inappropriate activation of the Wnt pathway was observed in many cancers, and it has been a target for anticancer therapy. The Wnt pathway can avoid its inappropriate activation through the proteolysis (degradation) of β-catenin with the involvement of WTX, APC, or Axin proteins. Tankyrases interfere in this function by inhibiting the activities of the Axin proteins and hence inhibit the degradation of β-catenin. Thus, the inhibition of tankyrases can regulate the Axin levels and increase degradation of β-catenin. |
| PARP inhibitors are known in the art. According to recent studies, PARP inhibitors interfere with DNA repair on various levels, which enhance the rate of cancer cell death. They also inhibit angiogenesis, either by inhibiting growth factor expression, or by inhibiting growth factor-induced cellular proliferative responses. In vivo studies showed that PARP inhibitors can nullify vascular endothelial growth factor (VEGF) or placental growth factor (PlGF)-induced migration, prevent formation of tubule-like networks, and weaken angiogenesis. Additionally, PARP-1 knockout mice have shown deficiency in growth factor-induced angiogenesis. Therefore, the inhibition of PARP1 and tankyrases is a novel therapeutic target that can lead to the development of useful treatments for cancer and many other disorders. The compounds described in this invention are inhibitors of TNKS-1 and TNKS-2 and may potentially be beneficial in developing a treatment of TNKS-induced diseases. |
| Important Compound Classes: |
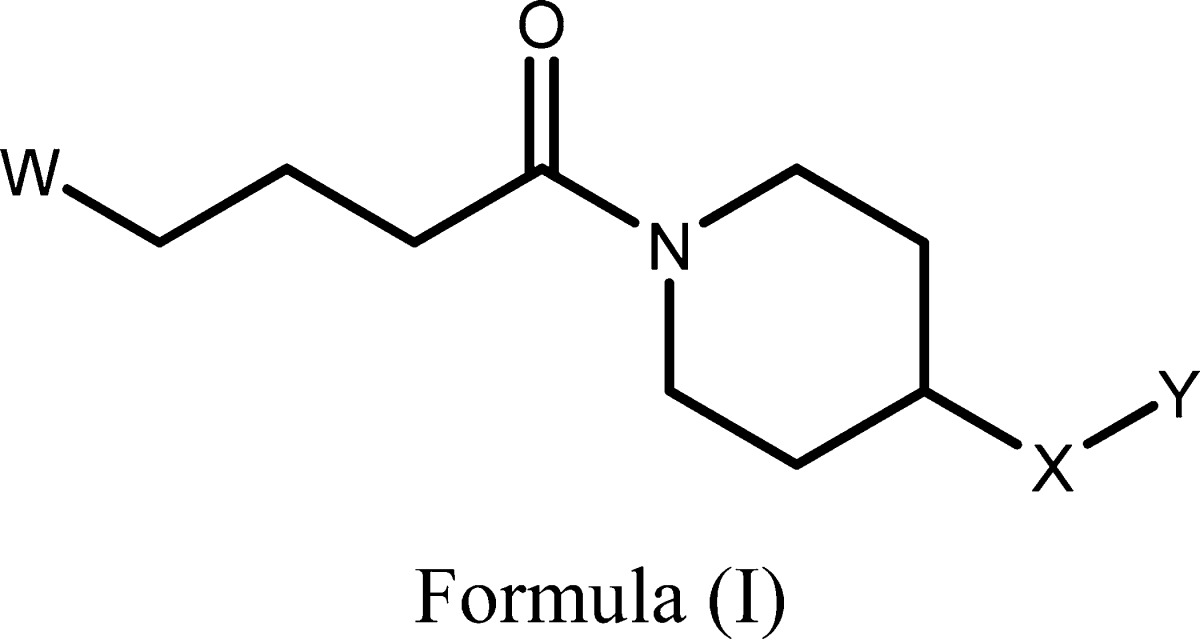 |
| Key Structures: |
The inventors described the synthesis and structures of 282 examples of formula (I) including the following compounds: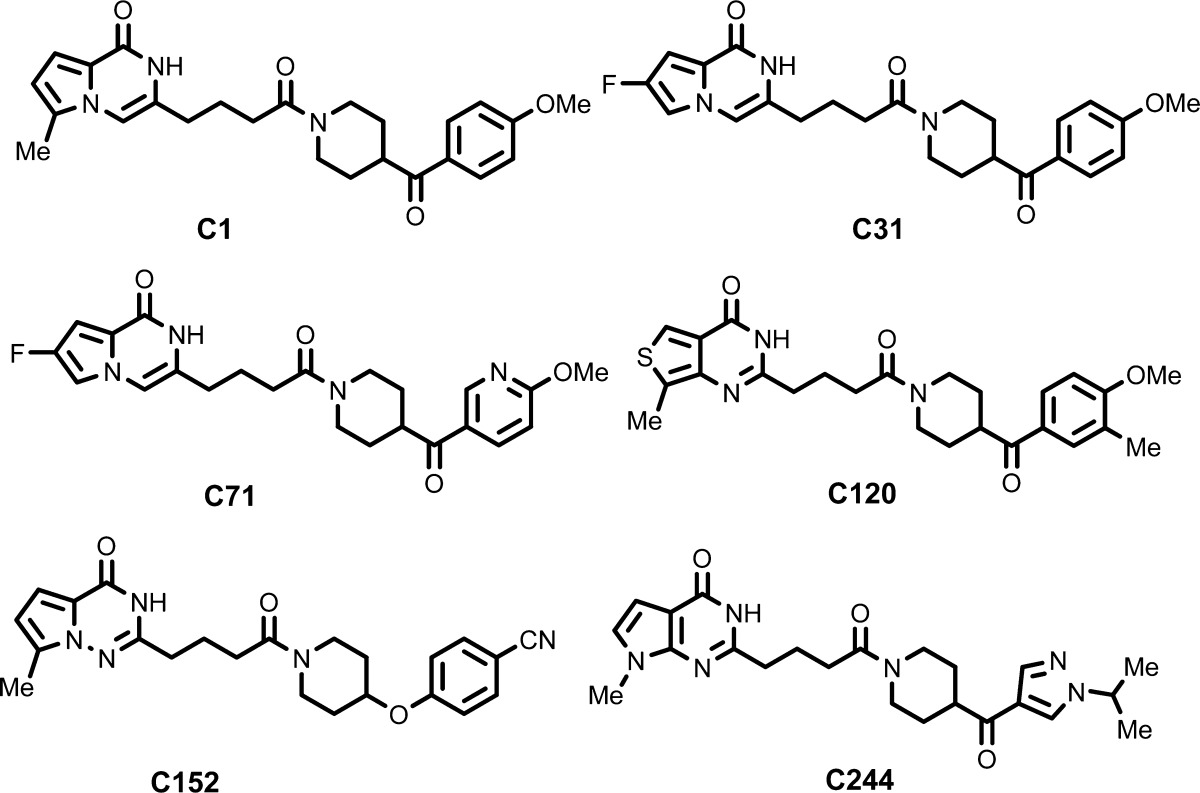
|
| Biological Assay: |
|
| Biological Data: |
The following table contains biological data obtained from testing the above representative examples: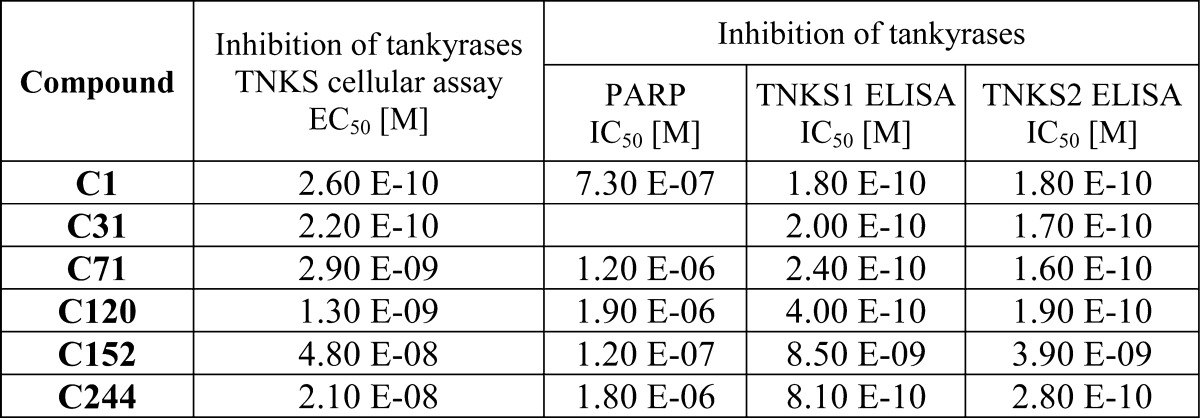
|
| Recent Review Articles: |
1. Buege M.; Mahajan P. B.. Rev. Recent Clin. Trials 2015, 10 ( (4), ), 326–339. |
| 2. Kamal A.; Riyaz S.; Srivastava A. K.; Rahim A.. Curr. Top. Med. Chem. 2014, 14 ( (17), ), 1967–197. |
| 3. Hottiger M. O.Expert Opin. Ther. Targets 2015, 19 ( (9), ), 1149–1152. |
| 4. Naipal K. A.; van Gent D. C.. Personal. Med. 2015, 12 ( (2), ), 139–154. |



