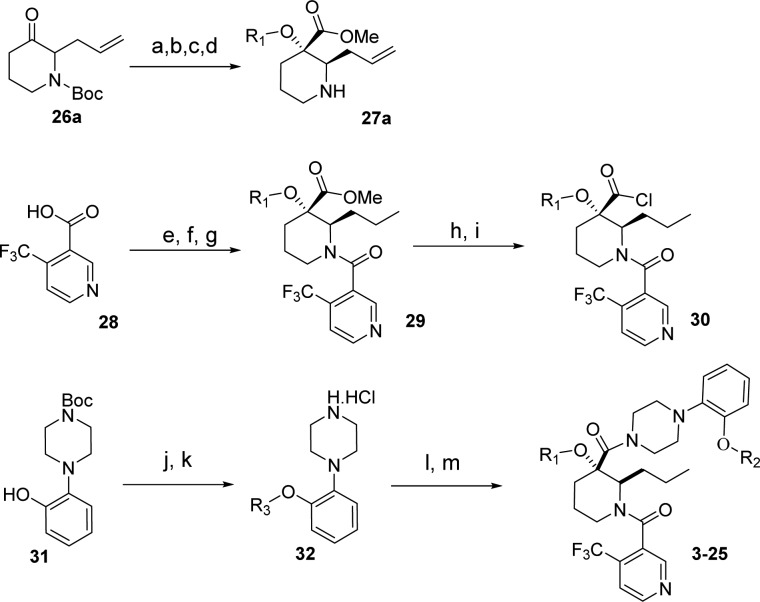
Scheme 1. Synthesis of 3–25 (General Method Method 1).
Reagents and conditions: (a) R1–OH, NaOH, CHCl3, 0–40 °C, 25–50%; (b) ACN/MeOH (1:1), 2.0 M TMSCHN, 20 °C, 0.5 h, 40–50%; (c) 50% TFA/DCM, 0 °C to rt, 3 h, (100% yield); (d) Chiralcel AD column, 90:10 heptanes/iPrOH; (e) benzene, thionyl chloride, reflux/cat DMF, 100%; (f) 27, DCM/DIPEA/0 °C, 100%; (g) H2, Pd/C, EtOH; (h) KOH, MeOH reflux, 100%; (i) (COCl)2, DMF, DCM 0 °C; (j) Cs2CO3, DMF, R3-Br (50–90% yield); (k) 4.0 N HCl dioxane 100%; (l) 30, THF/H2O, 0 °C to rt, 0.5 h (90–100% yield); (m) final deprotection step.