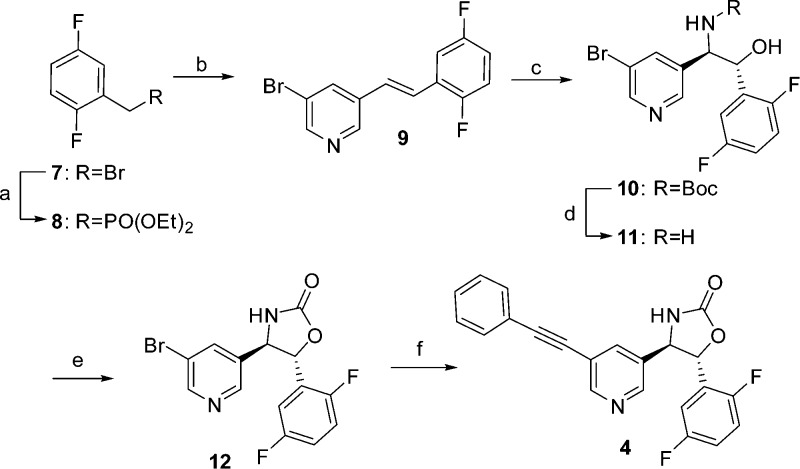
Scheme 1. Synthesis of 4 (BMS-955829).
Reagents and conditions: (a) P(OEt)3, 160 °C, 2–4 h, 93–100%; (b) 5-bromonicotinaldehyde, potassium t-butoxide, −10 °C, THF; (c) K2OsO4·2H2O, t-butylcarbamate, (DHQD)2PHAL, t-BuOCl, NaOH (0.5 M), H2O/propanol, 0 to 22 °C, 16 h, 32–35%; (d) HCl 4 M in dioxane, 50 °C, 3 h; (e) CDI, Hünig’s base, THF, 23–72%; (f) ethynylbenzene, PdCl2(PPh3)2, PPh3, CuI, TEA, reflux, 18 h, 60–85%.