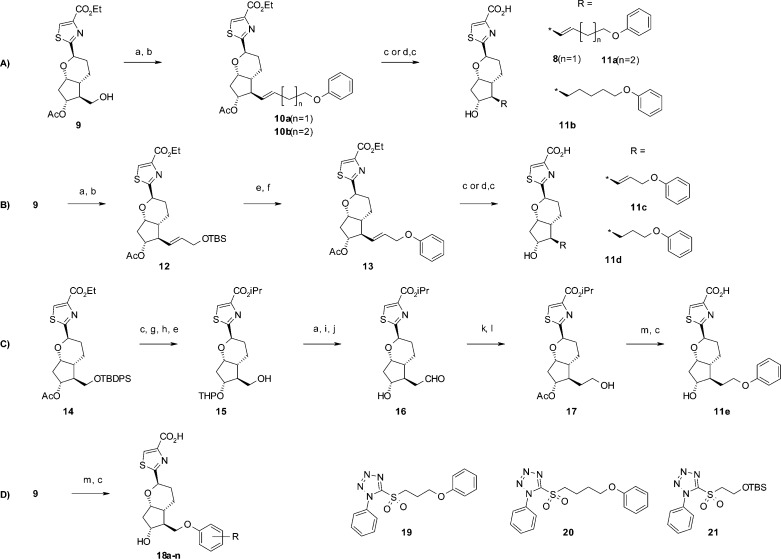
Scheme 1. Syntheses of Compounds 8, 11a–e, and 18a–n.
Reagents and conditions: (a) Dess–Martin periodinane, CH2Cl2, 0 °C, 77%; (b) 19, 20, or 21, KHMDS, DME, 0 °C, 18–66%; (c) 2 mol/L NaOHaq, DME, MeOH, rt, 56–96%; (d) TsNHNH2, NaOAc, EtOH, H2O, 80 °C, 55–71%, (e) TBAF, THF, rt, 96%; (f) DEAD, Ph3P, THF, rt, 82%; (g) i-PrI, K2CO3, DMF, rt, 54%; (h) PPTS, CH2Cl2, DHP, rt; (i) (methoxymethyl)triphenylphospine chrolide, KOtBu, THF, rt, 64%; (j) TsOH, acetone, H2O, rt, 78%; (k) Ac2O, Py, rt, 82%; (l) NaBH4, THF, rt, 61%; (m) phenol analogues, TMAD, Bu3P, THF, rt, 61–92%. Syntheses of common intermediate 9 and Julia–Kocienski reagents 19–21 are shown in the Supporting Information.