Abstract
Background
Unplanned readmissions to hospital are used in many healthcare systems as a quality indicator of care. Identifying patients at risk of readmission is difficult; existing prediction tools are only moderately sensitive. Correlations exist between certain medicines and emergency readmission, but it is not known whether the association is direct or indirect.
Objectives
To determine whether person-centred pharmaceutical care bundles, comprising individualised medicines information, risk management and/or support in taking medicines, might prevent unplanned readmissions by improving adherence and reducing avoidable harm from prescribed medications.
Methods
We designed and implemented person-centred pharmaceutical care bundles for patients who were socially isolated and/or on high-risk medicines on one older people's medical ward for 1 year from February 2013. Another ward with similar patient demographics, service characteristics and a standard clinical pharmacy service was used as a comparator in a prospective cohort study. Readmission rates were retrospectively studied for 12 months before the intervention and during the 12-month intervention period.
Results
The readmission rates for the intervention and control wards in the 12 months before the intervention were not significantly different. During the intervention period, the readmission rate was significantly lower on the intervention ward (69/418) than on the control ward (107/490; 17% vs 22%, p<0.05, z=2.05, two-sample z test for difference in proportions of unrelated samples).
Conclusions
Person-centred pharmaceutical care bundles were significantly associated with reduced risk of emergency hospital readmission in this study. Further evaluation of the model is warranted.
Keywords: CLINICAL PHARMACY, GERIATRIC MEDICINE, INDIVIDUALISED MEDICATION SURVEILLANCE, SOCIAL MEDICINE
Introduction
Unplanned readmission to hospital is used in many healthcare systems as a quality indicator because some will be the result of poor inpatient care or badly organised support or rehabilitation after discharge. The proportion of readmissions that are avoidable is difficult to specify as the criteria outlined in published studies, used to determine whether an admission was avoidable, are often subjective.1
As an incentive to improve inpatient care and discharge process quality, English National Health Service (NHS) acute care hospital Trusts face financial penalties as a consequence of emergency readmissions.2 The UK Department of Health, and thus the definition used in this paper, defines emergency readmissions as unplanned hospital admissions occurring within 30 days of the previous, last hospital discharge, but excluding admissions for malignant cancer, cancer chemotherapy or specialist obstetric or mental health services. Numbers of emergency readmissions are collected routinely in the UK as part of each Trust's Hospital Episode Statistics, the means by which Trusts are paid for the care they provide. The data are also used at a local and national level for resource management, information and clinical governance.
Clinicians and managers working to reduce readmissions in the UK face a number of challenges. The majority of the published evidence around hospital readmissions comes from health settings in the USA or Canada and may not be directly transferable to the UK.3 4 In addition, identifying patients at risk of readmission is difficult; healthcare and social care professionals are unable to accurately predict which individual patients will be readmitted5 6 and prediction tools for hospital readmission within 30 days have moderate sensitivity at best3 although certain prediction tools may be more discriminatory in particular patient populations.7 8 Although multiple hospital admissions are demonstrably associated with future admission,9 modelling with patient-specific factors such as medical conditions rather than epidemiological factors does not appear to increase the sensitivity of readmission prediction tools for medical patients;10 11 this suggests that the health belief model and health behaviours of individuals might be important factors in readmission. A further challenge lies in preventing the readmissions that have been predicted; staff resource is increasingly under pressure in the NHS because of ongoing cost-saving programmes but proven interventions tend to be resource intensive, particularly if conducted in the patient's home.3
Newcastle upon Tyne Hospitals NHS Foundation Trust (henceforth referred to as the Trust) is an 1800 bed teaching hospital in the North of England. Data from the Office for National Statistics show that our local population has some of the highest deprivation indices and lowest life expectancies in the UK. A 2012 audit of readmissions occurring in 1 week within the Trust identified medicines as a causal/contributing factor in 16 of 81 cases (20%). Twelve of those medication-related readmissions (75%) were judged by a multidisciplinary team to have been avoidable. Weak-to-moderate correlations can be found in the literature between certain medications and likelihood of readmission, namely, corticosteroids, psychoactive agents especially opioids, anti-epileptics, anti-neoplastics, immune-modulating agents, non-steroidal anti-inflammatory agents, anticoagulants, diuretics, drugs acting on the renin–angiotensin–aldosterone system and anti-diabetic agents including insulin,12–14 but it is not known whether those medicines are causal factors in readmission. The correlation may be an indirect association between those medications and long-term conditions such as heart failure or chronic obstructive pulmonary disease that are known to have high healthcare use and be associated with polypharmacy.
Polypharmacy (defined as five or more prescribed medicines),15 poor discharge planning,16 poor communication with the patient,17 or the primary care team,18 at transfer of care and inadequate follow-up by the hospital19–21 have been found in observational studies to result in adverse patient outcomes including unplanned or avoidable readmission.22 It is therefore plausible that the behaviours of healthcare professionals and/or patient-specific factors such as medical condition(s) and adherence to treatment(s) may be more accurate predictors of hospital readmission risk than the individual's epidemiological grouping.23 The majority of effective interventions identified from the literature were face-to-face and person-centred; five key themes emerged from our literature review and are shown in figure 1.
Figure 1.
Key themes identified as effective components in preventing readmissions. NMS, New Medicines Service; MUR, Medicines Use Reviews.
Our hypothesis was that person-centred pharmaceutical care during and after a hospital admission, that is meeting each individual's need for information, risk management or support to take their medicines, may reduce the rate of emergency readmissions caused by non-adherence or troublesome side effects. Traditionally, encounters between clinical pharmacists and patients have been pharmacist led with the goal of giving information. In our new pharmaceutical-care model, the encounters between the clinical pharmacists and patients were conversations led by patients where pharmacists spent more time listening than talking and the goal was to help the patient and carer share treatment decisions and find solutions to any problems identified. In addition, person-centred pharmaceutical care bundles were developed by the clinical pharmacy team for each of the medication types known to be correlated with readmission; two are shown as illustrative examples in figures 2 and 3. Existing national or local procedures for medication risk minimisation, such as National Patient Safety Agency (NPSA) guidance, were built into the care bundles.
Figure 2.
Person-centred pharmaceutical care bundle for opioids.
Figure 3.
Person-centred pharmaceutical care-bundle for corticosteroids. COPD, chronic obstructive pulmonary disease; NMS, New Medicines Service. FRAX is the name of a published tool, the FRAX(R) tool, available at https://www.shef.ac.uk/FRAX/tool.jsp
Although a large number of person-centred interventions designed to reduce emergency readmissions were identified in the literature, description or evaluation of the intervention was often vague or absent from the original articles. The clinical pharmacy team mapped the risks associated with a medical admission or transfer of care for older people, identified interventions that had demonstrated effectiveness for managing those risks (eg, Teach-back,24 careful medicine reconciliation, shared decision making, motivational interview techniques, real-time discharge communication, assessing a person's usual support network for suitability, providing person-centred information) and incorporated these into a patient-level document to be used alongside and to record completion of the pharmaceutical care bundles. A standard operating procedure (SOP) was chosen as the mechanism for introducing the care bundles to pharmacy practice because the pharmacy team was already familiar with the benefits of SOP on controlling variation in processes. A detailed description of the intervention, patient assessment tool and pharmaceutical care bundles can be accessed as an online supplementary file.
Hospital pharmacy staff are well placed to identify and address these needs during an admission, but are often not resourced or commissioned to provide domiciliary services after hospital discharge. The New Medicines Service (NMS) and targeted Medicines Use Reviews (MUR) are Community Pharmacy services designed to address a person's medication-related needs and are UK NHS funded in a broad range of circumstances including recent hospital discharge.25 The opportunity exists for hospital and community pharmacy teams to join-up to provide person-centred pharmaceutical care across the primary–secondary care interface.26
This paper describes the effect of introducing the intervention on an older people's medical ward in a prospective, cohort study design.
Methods
Ethical consideration
This was an observational study that assessed the effectiveness of a service improvement on readmission rather than implementing a new regimen of treatment and therefore full ethical approval was not required for this exercise. The study complies with the relevant principles of good practice in research and data protection.
The intervention and patient record
To maximise the chances of successful adoption by the pharmacy team, pharmacy staff working on the intervention ward were engaged as key stakeholders in the design and testing of the person-centred pharmaceutical care bundles, SOP and a patient-level document to record care-bundle completion.27 The patient-level documentation was designed according to the Trust standard format and refined after piloting to make it as quick and easy to complete as possible; a time–motion observation of the pharmacy technician working with five patients suggests that 10 min per patient was required to complete the document.
Each patient admitted to the intervention ward was screened by the pharmacy team as prescribed by the SOP and the pharmacy team implemented any relevant care bundles during the patient's stay and at discharge. Patients who had a longer stay on the ward were rescreened once a week using the SOP to identify any new medication-related risks or changes to discharge plans. Patients discharged from the intervention ward were offered a referral to their community pharmacy for NMS or MUR if they met the eligibility criteria; patients who met the eligibility criteria, but could not receive the service because they were housebound were offered the same services delivered by the hospital pharmacy team.
Description of the patients and pharmacy staff on the intervention and control wards
Two wards specialising in the care of medical patients over 80 years of age with similar service statistics (Healthcare Resource Groups28 and average length of stay) were identified for the study; one was selected at random to be the intervention ward and patients were consequently allocated non-experimentally to either the intervention or control ward by the hospital bed management team who were unaware that a study was taking place. Both wards already had a named pharmacist for 5 days per week who performed medicines reconciliation after admission, some patient education and clinical medication review. Fifteen hours per week of pharmacy technician time was allocated to the intervention ward to deliver the intervention in conjunction with the pharmacist. To minimise confounding, the pharmacy technician did not work on the control ward and pharmacists working on the control ward were prevented from seeing the person-centred pharmaceutical care bundles or the individual patient checklist.
Data and variables
Readmission rates and average length of stay were obtained for each ward from the Trust Informatics Department; these are routinely collected monthly hospital activity data. The data for pharmaceutical care bundle completion rate for the intervention period and the numbers of patients requiring a home visit or telephone call after discharge were retrospectively collected from each patient-level record card. Medicine reconciliation rate was audited biannually for routine performance reports by a pharmacist unrelated to the study. Patient and general practitioner (GP) feedback was captured by self-administered postal questionnaire over 2 months of the intervention period; the questionnaires were deliberately short and contained a first-class stamped, addressed envelope to help secure a good response rate. The data were analysed by a researcher with statistical expertise. As this was a natural experiment without randomisation and with an unknown effect size, formal sample size calculation was not considered. The data obtained from this study will help to power a future trial. A 12-month intervention period was selected to evaluate sustainability of the model and eliminate the possibility of confounding by seasonal variations in readmission rate.
Analysis
Using summary data for before and during intervention period, estimates of risk difference, OR were estimated by a researcher with statistical expertise. Breslow–Day and Tarone's statistics test was used to test for homogeneity of OR from during and before intervention periods. Fisher's exact test was used to analyse available data for intervention period to examine whether proportion of readmission varied by the different components of treatment plan. The analysis was done separately for intervention and control ward, as well as for examining overall (combined for both ward) difference in proportion readmitted by the different components. Logistical regression analysis was done to examine whether readmission rate varied significantly between intervention and control wards and also to identify variables that were significantly associated with readmission (control for other variables). IBM SPSS Statistics, V.20 was used to analyse the data. The qualitative data from patient and GP questionnaires are simply described.
Results
Outcome measures
During the intervention, readmission rate was 5% lower in intervention ward in comparison with that of the control ward (table 1). The odds of readmission for the control ward was 41% higher than the odds of readmission for the intervention ward during the intervention period (OR=1.41, 95% CI 1.01 to 1.98). However, the control ward had lower odds of readmission than the intervention ward at baseline (OR=0.82, 95% CI 0.56 to 1.14). The Breslow–Day and Tarone's test examined the homogeneity of OR between the two periods and found that they were significantly different. This qualitative interaction suggests that the intervention may have a positive impact on hospital readmission. Therefore, the subsequent analyses were done for the data obtained during the intervention period only to identify risk factors for hospital readmissions.
Table 1.
Readmissions within 30 days for the intervention and control wards before and during the intervention
| Control ward | Intervention ward | Two sample z-test for difference in population proportions | |||
|---|---|---|---|---|---|
| Patient numbers | Discharges | Readmissions | Discharges | Readmissions | |
| 12-month period prior to intervention | 446 | 72 (16.1%) | 403 | 78 (19.4%) | Not significantly different |
| 12-month intervention period | 490 | 107 (21.8%) | 418 | 69 (16.5%) | z=2.05, p=0.041 |
| Pearson's goodness-of-fit χ2 test for difference in proportions | Not significantly different χ2=3.33, p=0.068 |
Not significantly different χ2=1.52, p=0.375 |
|||
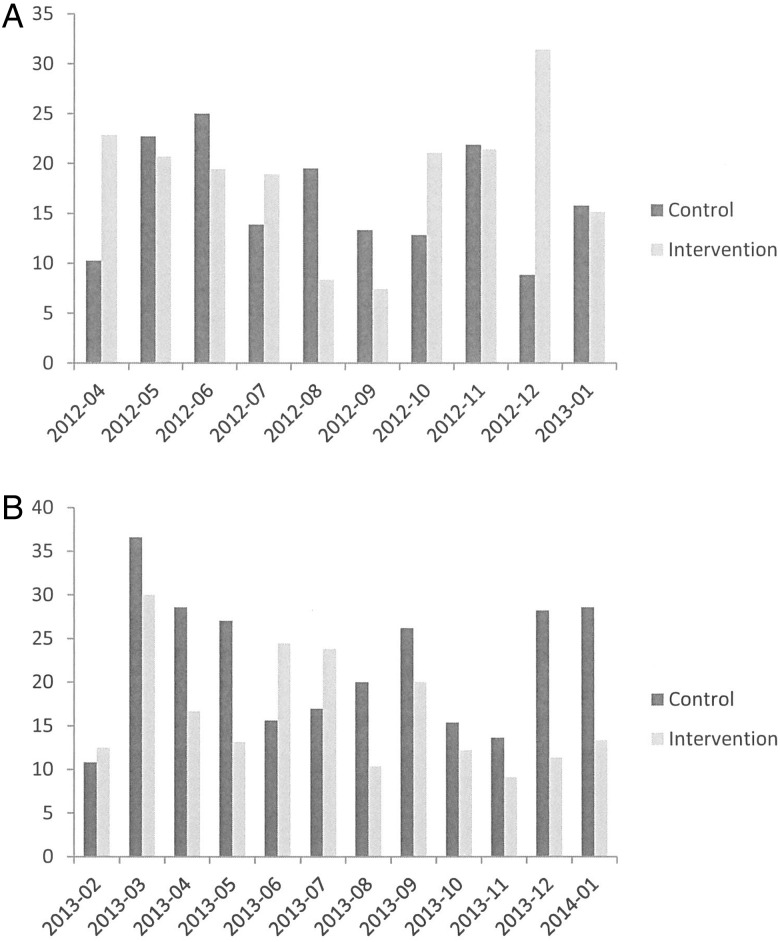
Figure 4A shows the variability in monthly readmission rates for the intervention and control wards in the months before the intervention; figure 4B shows the same parameters during the intervention period; the intervention ward has lower readmission rates than the control ward for 10 of the 12 months of the intervention period.
Figure 4.
Readmission rate (%) for the intervention and control wards before (A) and during (B) the intervention.
Average length of stay did not change significantly on either ward during the intervention period. Medicines reconciliation after admission was recorded for 100% of patients sampled on both the intervention and control wards in both biannual audit periods.
In the intervention ward, readmission was significantly associated with monitored dosage system (MDS) use (readmission=16.8% and 7.4% among service users vs non-service users, respectively; risk difference=9.4%; p=0.03) and whether the discharge medication list was sent in real time or not (22.7% vs 7.9% among service users and non-service users, respectively; risk difference=14.8%; p<0.001). ORs for these factors are shown in table 2. However, there was no significant difference in readmission rate for the other treatment components (all p>0.05). Multivariable logistical regression analysis showed that controlling for other factors (MDS use, requirements of self-medication assessment, sending discharge medicine in real time), the likelihood of patients being readmitted was less where pharmacy staff completed actions prescribed by the SOP compared with patients who were discharged before the prescribed actions were completed (OR=0.539, 95% CI 0.299 to 0.969; p=0.039).
Table 2.
Multivariate logistical regression analysis examining factors associated with readmission
| Indicators | OR | 95% CI | |
|---|---|---|---|
| Lower | Upper | ||
| Intervention effect (ward) | 0.539 | 0.299 | 0.969 |
| Uses MDS | 1.619 | 0.903 | 2.905 |
| Self-medication assessment required | 0.494 | 0.271 | 0.898 |
| Discharge medication list sent in real time | 2.423 | 1.359 | 4.322 |
| Constant | 0.166 | ||
MDS, monitored dosage system.
An average of three patients per month required follow-up after discharge by the hospital pharmacy team. Ninety per cent of follow-up was possible by telephone and the telephone calls typically lasted around 5 min. Home visits typically lasted around 20 min plus travelling time. All patients who replied to our postal questionnaire (13 respondents, response rate 43%) accepted the telephone follow up or home visits from hospital pharmacy staff and 12 of 13 (92%) reported that they found the pharmacy technician's advice helpful. We referred an average of 15 patients per month to community pharmacies for NMS or MUR, but uptake of those services was not monitored. Thirty GPs (response rate 71%) replied to our postal questionnaire. Approximately half (14, 47%) of the GP respondents agreed that the discharge information we provided about medicines was more accurate and timely than they would typically receive.
Discussion
Our results suggest that the risk of medication-related readmissions can be reduced with person-centred pharmaceutical care and that pharmaceutical care bundles are a practicable way to introduce person-centred care with medicines to an acute teaching hospital in the UK NHS.
Our study has several limitations relating to the design and conduct. For pragmatic reasons, our primary outcome measure was obtained from clinical coding data, which is known to contain inaccuracies; a recent audit conducted on behalf of the UK Department of Health identified wide inter-Trust coding error variation with an average error rate of 7%.29 Patients were not involved in the design of the study and their perspectives may have caused us to alter the design or delivery of the intervention. As a cohort study, there may be factors influencing readmission rate that we cannot measure or control. For example, the two wards in our study have different lead clinicians; those clinicians may impact the culture of the ward and subsequently on discharge planning and readmissions, but that effect would be difficult to quantify objectively. Loss to follow-up can be a difficulty in cohort studies, but was not a major issue in this study. We hypothesise that this is the result of the need for planned discharge in this patient group, which typically occurs within usual working hours because support services are often unavailable outside those hours. Only one patient was transferred from the intervention to the control ward in the 12-month intervention period, those data have not been excluded from our results, but are unlikely to have an effect on our primary outcome measure. We did not examine the sociodemographic or socioeconomic characteristics of patients on the intervention or control wards; although there is no reason to expect a difference, this potentially confounds our results.
The apparent reduction of readmission rate in our study should be received cautiously for the reasons described above and because the study was not powered to detect this difference. The prolonged period where the readmission rate for the intervention ward was lower than control ward (figure 4B) is unusual behaviour for readmission rates and suggests that the intervention was having an effect. Interestingly, the magnitude of the observed difference is similar to that observed in Leeds Teaching Hospitals IMPACT study where hospital pharmacy joined up with community pharmacy at transition of care in a similar patient group.30
The association between MDS use and increased risk of readmission observed in our study is interesting and warrants further investigation. The association may be the indirect result of the characteristics of patients who are provided with MDS (often people vulnerable to adverse drug events because of polypharmacy or cognitive impairment) or the direct result of MDS removing a link between patients and their medicines. The association we observed between real-time discharge communication and increased risk of readmission is possibly also a reflection of the impact of MDS use; the intervention SOP directed pharmacy staff to real-time discharge communication for patients with MDS and/or district nurse support with insulin to reduce dosing errors as a result of changes made in hospital.
The wards chosen for this study are typical of acute medical wards for older people in UK teaching hospitals but our results may not be transferable outside this speciality.
Key messages.
What is already known on this subject
Readmission risk prediction models are widely used but not sensitive.
A number of medications are correlated with readmission but a causal link is not established.
What this study adds
Person-centred risk assessment and risk management for older people and their medications in hospital may reduce the likelihood of 30-day readmission by 40%.
Using a monitored dosage system for medicines at home may be a significant risk factor for hospital readmission.
Acknowledgments
Karen Glasper and Christine Baty, Pharmacy Technicians at Newcastle upon Tyne Hospitals NHS Foundation Trust. David Grainger, General Practitioner, Newcastle West Clinical Commissioning Group. Adetayo Kasim, Research Statistician, Wolfson Research Institute for Health and Wellbeing, for providing support with statistical analysis.
Footnotes
Twitter: Follow Julia Blagburn at @juliablagburn
Contributors: Planning of the study including literature search and design of the intervention and data collection tools was done by JB and BK-F. The study was conducted by JB and KG. Statistical design and analysis was done by NA and AK. JB drafted the article which was revised by AH, BK-F, DG and AK.
Funding: Newcastle West Clinical Commissioning Group supported this study by funding a Pharmacy Technician for 15 h per week for 1 year.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Anonymised data are available on request from the corresponding author.
References
- 1.van Walraven C, Bennett C, Jennings A, et al. . Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ 2011;183:E391–402. 10.1503/cmaj.101860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Department of Health Payment by Results team (2012). A simple guide to Payment by Results. London: Department of Health, Gateway reference 18135.
- 3.Kansagara D, Englander H, Salanitro A, et al. . Risk prediction models for hospital readmission: a systematic review. JAMA 2011;306:1688–98. 10.1001/jama.2011.1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The King's Fund. Avoiding hospital admissions. What does the research evidence say? http://www.kingsfund.org.uk/sites/files/kf/Avoiding-Hospital-Admissions-Sarah-Purdy-December2010_0.pdf (accessed 4 Dec 2012).
- 5.Allaudeen N, Schnipper JL, Orav EJ, et al. . Inability of providers to predict unplanned readmissions. J Gen Intern Med 2011;26:771–6. 10.1007/s11606-011-1663-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Toole R, Campbell PMF. Predicting readmissions of patients from an elderly care ward. Age Ageing 2012;41:0002–729. 10.1093/ageing/afs126 [DOI] [Google Scholar]
- 7.Cotter PE, Bhalla VK, Wallis SJ, et al. . Predicting readmissions: poor performance of the LACE index in an older UK population. Age Ageing 2012;41:784–9. 10.1093/ageing/afs073 [DOI] [PubMed] [Google Scholar]
- 8.Walsh C, Hripcsak G. The effects of data sources, cohort selection and outcome definition on a predictive model of risk of thirty-day hospital readmissions. J Biomed Inform 2014;52:418–26. 10.1016/j.jbi.2014.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baillie CA, VanZandbergen C, Gordon H, et al. . The readmission risk flag: using the electronic health record to automatically identify patients at risk for 30-day readmission. J Hosp Med 2013;8:689–95. 10.1002/jhm.2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donzé J, Aujesky D, Williams D, et al. . Potentially avoidable 30-day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Int Med 2013;173:632–8. 10.1001/jamainternmed.2013.3023 [DOI] [PubMed] [Google Scholar]
- 11.Taha M, Pal A, Mahnken JD, et al. . Derivation and validation of a formula to estimate risk for 30-day readmission in medical patients. Int J Qual Health Care 2014;26:271–7. 10.1093/intqhc/mzu038 [DOI] [PubMed] [Google Scholar]
- 12.Avery RL, Slavenburg S, Royal S, et al. . Which drugs cause preventable admissions to hospital? A systematic review. Br J Clin Pharmacol 2006;63:136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu TY, Jen MH, Bottle A, et al. . Ten-year trends in hospital admissions for adverse drug reactions in England 1999–2009. J R Soc Med 2010;103:239–50. 10.1258/jrsm.2010.100113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pavon JM, Zhao Y, McConnell E, et al. . Identifying risk of readmission in hospitalized elderly adults through inpatient medication exposure. J Am Geriatr Soc 2014;62:1116–21. 10.1111/jgs.12829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sehgal V, Bajwa SJ, Sehgal R, et al. . Polypharmacy and potentially inappropriate medication use as the precipitating factor in readmissions to the hospital. J Family Med Prim Care 2013;2:194–9. 10.4103/2249-4863.117423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boling PA. Managing post-hospital care transitions for older adults: challenges and opportunities. JAMA 2014;312:1303–4. 10.1001/jama.2014.12360 [DOI] [PubMed] [Google Scholar]
- 17.Pal A, Babbott S, Wilkinson ST. Can the targeted use of a discharge pharmacist significantly decrease 30-day readmissions? Hosp Pharm 2013;48:380–8. 10.1310/hpj4805-380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witherington EMA, Pirzada OM, Avery AJ. Communication gaps and readmissions to hospital for patients over 75 years and older: an observational study. Qual Saf Health Care 2008;17:71–5. 10.1136/qshc.2006.020842 [DOI] [PubMed] [Google Scholar]
- 19.Benbassat J, Taragin M. Hospital readmissions as a measure of quality of health care: Advantages and limitations. AMA Arch Intern Med 2000;160:1074–81. 10.1001/archinte.160.8.1074 [DOI] [PubMed] [Google Scholar]
- 20.Hernandez AF, Greiner MA, Fonarow GC, et al. . Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA 2010;303:1716–22. 10.1001/jama.2010.533 [DOI] [PubMed] [Google Scholar]
- 21.Seymour JM, Moore C, Jolley CJ, et al. . Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax 2010;65:423–8. 10.1136/thx.2009.124164 [DOI] [PubMed] [Google Scholar]
- 22.Rennke S, Nguyen OK, Shoeb MH, et al. . Hospital-initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med 2013;158(5 Pt 2):433–40. 10.7326/0003-4819-158-5-201303051-00011 [DOI] [PubMed] [Google Scholar]
- 23.Lau DT, Nau DP. Oral antihyperglycemic medication nonadherence and subsequent hospitalization among individuals with type 2 diabetes. Diabetes Care 2004;27:2149–53. 10.2337/diacare.27.9.2149 [DOI] [PubMed] [Google Scholar]
- 24.Schillinger D, Piette J, Grubach K, et al. . Closing the loop: physician communication with diabetic patients who have low health literacy. AMA Arch Int Med 2003;163:83–90. 10.1001/archinte.163.1.83 [DOI] [PubMed] [Google Scholar]
- 25.Department of Health. The pharmaceutical services (advanced and enhanced services) (England) directions. London: Department of Health, 2005. [Google Scholar]
- 26.Blenkinsopp A, Bond C, Celino G, et al. . Medicines use review: Adoption and spread of a service innovation. Int J Pharm Pract 2008;16:271–6. 10.1211/ijpp.16.4.0010 [DOI] [Google Scholar]
- 27.Cameron E, Green M. Making sense of change management: a complete guide to the models, tools and techniques of organizational change. London, UK: Kogan Page Limited, 2004:48–54. [Google Scholar]
- 28.Department of Health. National Casemix Office: Introduction to Healthcare Resource Groups. http://www.hscic.gov.uk/hrg (accessed Oct 2014).
- 29.Caspe Healthcare Knowledge Systems: The quality of clinical coding in the NHS. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/364476/The_quality_of_clinical_coding_in_the_NHS.pdf (accessed Apr 2015).
- 30.Smith H, Fox G, Khan I, et al. . Review of patients readmitted post IMPACT pharmacist intervention designed to reduce medicines-related re-admissions[abstract]. Age Ageing 2014;43(Suppl 1):i17. [Google Scholar]