Summary
Background
Matrix metalloproteinases (MMPs) are upregulated in tumors. The MMP-7 A-181G polymorphism is associated with increased expression of the MMP-7 gene. Aim of the present study was to investigate the association between the MMP-7 A-181G polymorphism and susceptibility to breast cancer.
Patients and Methods
The MMP-7 A-181G variants were studied in a cohort of 251 subjects consisting of 100 breast cancer patients and 151 healthy controls; all were from Western Iran. The MMP-7 A-181G genotypes were identified using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis.
Results
The frequencies of the MMP-7 AA, AG, and GG genotypes in healthy individuals were 34.4, 50.4, and 15.2%, respectively. In breast cancer patients, the frequencies of AA (34%), AG (52%), and GG (14%) genotypes (p = 0.95) were similar to those in the controls. There was a trend toward an increased frequency of the combined genotype of MMP-7 AG+GG in patients with lymph node metastasis (70.4%) compared to those without metastasis (66.7%). Also, in patients with invasive lobular carcinoma, the frequency of the MMP-7 AG+GG genotype tended to be higher (71.4%) compared to that in patients with invasive ductal carcinoma (66.2%) (p = 0.78).
Conclusion
Our findings indicate that the MMP-7 A-181G polymorphism may not be correlated with susceptibility to breast cancer in our population.
Keywords: Breast cancer, MMP-7 A-181G, Gene polymorphism, Western Iran
Introduction
Breast cancer is the most common cancer among women and is one of the common health problems in women from different populations. Breast cancer is associated with high incidence of morbidity and mortality throughout the world [1,2,3]. A medium prevalence of breast cancer has been reported among Iranian women with increasing incidence [4]. Both genetic and environmental risk factors and their interaction might be involved in the pathogenesis of breast cancer. Studies from various populations indicate that genetic factors including gene polymorphisms and the presence of mutations might be strong risk factors that influence the individual differences in susceptibility to breast cancer [5].
Matrix metalloproteinases (MMPs) are a family of zinc-dependent protease enzymes that degrade and restructure the extracellular matrix (ECM) components and basal lamina which are physical barriers for cancer cells [6,7,8]. MMPs have a broad substrate activity including the ECM components of collagen type I-VIII, gelatin, elastin, laminin, and also myelin basic protein and several growth factors. Generally, in healthy individuals, there is a low level of MMPs. However, in human cancers of all stages of carcinogenesis, higher expression of these enzymes has been detected [9], which could potentially affect tumor grade and metastasis. Overexpression of MMP genes, through remodeling of the ECM, might increase the invasive potential of cancer cells [6].
Individual susceptibility to breast cancer might be affected by alterations in MMP gene expression [6,10]. MMP-7 is an important member of the MMP gene family with broad substrate specificity against both ECM and non-ECM components [11]. The MMP-7 gene is located on chromosome 11q21-q22, and has 13 exons. The A-181G (rs11568818) polymorphism in the promoter region of the MMP-7 gene modulates gene transcription through affecting the binding of nuclear protein(s) [12,13]. Nuclear proteins bind to the MMP-7 −181G allele with higher affinity compared to the −181A allele. Hence, in the presence of the −181G allele, the promoter activity is 2-3 times higher compared to the −181A allele [12]. Some studies have demonstrated that breast cancer is associated with higher MMP-7 gene expression [9,14].
According to the literature, there are no available studies that have examined the association between the MMP-7 A-181G polymorphism and breast cancer susceptibility in Iranian populations. The aim of the present study was to investigate the frequency of MMP-7 A-181G variants and their association with breast cancer risk among an Iranian population of Kurdish ethnic background.
Patients and Methods
Sample
The sample in the present case control study comprised 100 breast cancer patients including 99 females and 1 male with a mean age of 49.5 ± 10.2 years (range 29-79 years) and 151 healthy females with a mean age of 38.7 ± 9.4 years (range 22-68 years). The breast cancer diagnosis was made according to standard clinical, radiological, and histological parameters. All patients were from the Kermanshah and Ilam provinces of Iran and were of Kurdish ethnic background, and had been admitted to the Kermanshah University of Medical Sciences hospital. Controls had the same ethnic background as patients. Demographic and medical characteristics of patients including age, sex, family history of cancer, tumor stage, lymph node metastasis, and the status of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor (HER2), P53, and KI67 were obtained from the patient files. The study procedures were approved by the Ethics Committee of Kermanshah University of Medical Sciences, Iran. All patients and controls agreed to participate in the study and signed an informed written consent form in accordance with the principles laid down in the Helsinki II declaration.
Genotyping
A sample of 5 ml EDTA-treated whole blood was taken from each individual. Genomic DNA was extracted from peripheral blood leukocytes according to the phenol-chloroform method as previously described [15,16]. Using agarose gel electrophoresis (1%), the presence of extracted DNA was confirmed. The concentration and purity of DNA was assessed using a NanoDrop™ spectrophotometer (Thermo Fisher Scientific, Waltham, Massachusetts, USA).
The MMP-7 A-181G polymorphism was identified using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis. The PCR was conducted using the forward primer of 5'-TGGTACCATAATGTCCTGAATG-3' and the reverse primer of 5'-TCGTTATTGGCAGGAAGCACACAATGAATT-3’. The PCR thermal cycling parameters were: 1 cycle at 94 °C for 5 min, 35 cycles at 94 °C for 60 s, 62 °C for 60 s, and 72 °C for 60 s followed by final extension for 10 min at 72 °C. In the presence of G allele, the obtained PCR product with 150 bp was digested with EcoRI restriction enzyme into 2 fragments of 120 and 30 bp, respectively. The PCR product remained intact in the presence of A allele [17].
Statistics
The allelic frequencies were calculated using the chromosome counting method. The significance of the difference of alleles and genotype frequencies between the groups was tested using the chi-square method. A two-tailed Student's t test analysis was used to compare quantitative data, and these data were expressed as means ± standard deviations. Statistical significance was assumed at the p < 0.05 level. All of the statistical analyses were performed using the SPSS statistical software package, version 16.0 (IBM Corp., Armonk, NY, USA).
Results
Demographic, biochemical, and clinical characteristics of the study population are shown in table 1. 89% of patients had invasive ductal carcinoma, 9.8% had invasive lobular carcinoma, and 1.2% had ductal carcinoma in situ. Based on available immunohistochemical data, of the 100 breast cancer patients, 64 (71.2%) patients were ER-positive and 26 (28.8%) were ER-negative. PR positivity was detected in 65 (72.3%) patients; 25 (27.7%) patients were PR-negative. The frequency of other immunohistochemical markers including HER2+, P53+, and Ki67+ was 59.8, 42, and 94.5%, respectively (table 1).
Table 1.
Characteristics of breast cancer patients and controls
| Variable | Patients (n = 100) | Controls (n = 151) |
|---|---|---|
| Median age ± standard deviation (range), years | 49.5 ± 10.2 (29–79) | 38.7 ± 9.4 (28–70) (p< 0.001) |
| n (%) | ||
| Sex | ||
| Female | 99 (99) | 151 (100) |
| Male | 1 (1) | 0 |
| Family history of cancer | ||
| No | 53 (50.5) | – |
| Yes | 47 (49.5) | – |
| Stage | ||
| I | 13(19.7) | – |
| II | 42(63.6) | – |
| III | 11(16.7) | – |
| Lymph node metastasis | ||
| No | 21 (28) | – |
| Yes | 54 (72) | – |
| Histological Types | ||
| In situ | 1 (1.2) | – |
| Invasive ductal carcinoma | 73 (89) | – |
| Invasive lobular carcinoma | 8 (9.8) | – |
| Estrogen receptor | ||
| Positive | 26 (28.8) | – |
| Negative | 64 (71.2) | – |
| Progesterone receptor | ||
| Positive | 25 (27.7) | – |
| Negative | 65 (72.3) | – |
| HER2 | ||
| Positive | 33 (40.2) | – |
| Negative | 49 (59.8) | – |
| P53 | ||
| Positive | 42 (60) | – |
| Negative | 28 (40) | – |
| KI67 | ||
| Positive | 4 (5.5) | – |
| Negative | 70 (94.5) | – |
The agarose gel electrophoresis pattern of some RFLP products of the amplified MMP-7 gene digested with EcoRI is demonstrated in figure 1. The frequencies of MMP-7 genotypes were in Hardy-Weinberg equilibrium in both patients and controls (χ2 = 0.31 and χ2 = 0.69, respectively) (p > 0.1).
Fig. 1.
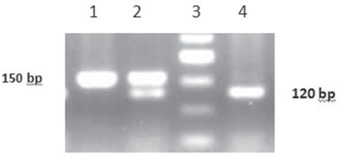
Agarose gel electrophoresis (3%) pattern of restriction enzyme (EcoRI)-digested polymerase chain reaction products. From left to right, lane 1 shows the wild genotype of MMP-7 AA, lane 2 demonstrates the heterozygous MMP-7 AG genotype, and lane 4 indicates mutant genotype of MMP-7 GG. The 50-bp DNA molecular weight marker is indicated in lane 3.
The distribution of MMP7 genotypes in breast cancer patients and controls is depicted in table 2. The frequencies of MMP-7 AA, AG, and GG genotypes in healthy individuals were 34.4, 50.4, and 15.2%, respectively. In breast cancer patients, similar frequencies were observed for the AA (34%), AG (52%), and GG (14%) genotypes (p = 0.95). Logistic regression models were created to examine various genetic mechanisms (table 2). As indicated in table 2, in the dominant genetic model (AG+GG vs. AA), the frequency of the AG+GG genotype was similar between patients (66%) (p = 0.94) and controls (65.6%). Further, in the overdominant genetic model (AG vs. AA+GG), the frequency of the AG genotype was non-significantly higher in patients (52%) compared to that in controls (50.3%). However, in the recessive genetic model (GG vs. AG+AA), there was a non-significantly higher frequency of GG genotype in controls (15.2%) (p = 0.78) compared to patients (14%). The frequency of the mutant allele of MMP-7 G was 41% in controls and 40% in patients (p = 0.82). Although the frequency of the combined genotype of MMP-7 AG+GG was higher in patients with lymph node metastasis (70.4%) than in those without metastasis (66.7%), this did not reach statistical significance (p = 0.75). The frequency of the combined genotype of MMP-7 AG+GG was higher (70.3 and 69.2%, respectively) in ER- and PR-positive patients compared to that in ER- and PR-negative patients (65.4% (p = 0.64) and 68% (p = 0.91), respectively). Also, in patients with invasive lobular carcinoma, the frequency of this genotype tended to be higher (71.4%) compared to that in patients with invasive ductal carcinoma (66.2%) (p = 0.78) (table 3).
Table 2.
Distribution of MMP-7 A-181G genotypes in breast cancer patients and controls
| Genotype MMP-7 A-181G | Patients, n (%) (n = 100) | Controls, n (%) (n = 151) | p value (χ2) |
|---|---|---|---|
| Codominant | |||
| AA | 34 (34) | 52 (34.4) | |
| AG | 52 (52) | 76 (50.3) | 0.87 (0.025) |
| GG | 14 (14) | 23 (15.2) | 0.86 (0.031) |
| Dominant | |||
| AA | 34 (34) | 52 (34.4) | |
| AG+GG | 66 (66) | 99 (65.6) | 0.94 (0.005) |
| Recessive | |||
| AG+AA | 86 (86) | 128 (84.8) | |
| GG | 14 (14) | 23 (15.2) | 0.78 (0.073) |
| Over dominant | |||
| AA+GG | 48 (48) | 75 (49.7) | |
| AG | 52 (52) | 76 (50.3) | 0.79 (0.067) |
| MMP-7 alleles | |||
| A | 120 (60) | 180 (59.6) | |
| G | 80 (40) | 122 (40.4) | 0.92 (0.008) |
Table 3.
Frequency of MMP-7 genotypes in patients according to clinical parameters
| Parameter | MMP-7 AA, n (%) | MMP-7 AG+GG, n (%) | p value (χ2) |
|---|---|---|---|
| Lymph node metastasis | |||
| No | 16 (29.6) | 38 (70.4) | 0.75 (0.098) |
| Yes | 7 (33.3) | 14 (66.7) | |
| Estrogen receptor | |||
| Positive | 9 (34.6) | 17 (65.4) | 0.64 (0.2) |
| Negative | 19 (29.7) | 45 (70.3) | |
| Progesterone receptor | |||
| Positive | 8 (32) | 17 (68) | 0.91 (0.013) |
| Negative | 20 (30.8) | 45 (69.2) | |
| Histology | |||
| In situ | 1 (100) | 0 (0) | 0.78a (0.078) |
| Invasive ductal carcinoma | 26 (33.8) | 51 (66.2) | 0.37b (2.031) |
| Invasive lobular carcinoma | 2 (28.6) | 5 (71.4) | |
Compared with invasive lobular carcinoma.
Overall.
Discussion
The present study among a homogenous population of breast cancer patients from Western Iran found the absence of a significant association between the MMP-7 A-181G polymorphism and the risk of breast cancer. However, we detected a trend toward a higher percentage of lymph node metastasis, invasive lobular carcinoma, and ER-positive status in the presence of the combined genotype of MMP-7 AG+GG.
Breast cancer is the most frequent malignancy to affects the health and lives of women in both developed and developing countries. Breast cancer is the third most common cause of death among Iranians after coronary heart disease and accidents [18]. According to epidemiological studies, a bulk of risk factors including genetic, epigenetic, and environmental factors are involved in the pathogenesis of breast cancer [9]. A number of functional single nucleotide polymorphisms have been identified in the promoter region of the MMP-7 gene with some of them having allele-specific effects on the regulation of MMP-7 gene expression and being associated with the development and progression of colorectal, ovarian, and gastric cancers [13,19]. The MMP-7 A-181G polymorphism influences the binding of nuclear proteins and promoter activity, thus modulating gene expression [20,21]. Higher promoter activity (2-3 times) in the presence of the MMP-7 −181G allele compared to the −181A allele in U937 cells has been attributed to the presence of a putative binding site (NGAAN) for the heat shock transcription factor in the MMP-7 −181G allele that is absent in the −181A allele [22]. There are inconsistent reports related to the role of MMP-7 A-181G variants in cancer development. An association of the MMP-7 A-181G polymorphism with esophageal squamous cell carcinoma, adenocarcinoma of the gastric cardia, and non-small cell lung cancer has been reported in the Han Chinese population [12]. Also, the MMP-7 −181G allele has been found to be prevalent among colorectal cancer patients [23]. Further, in a study among Caucasians, an association between this allele and reduced risk of gastric cancer has been demonstrated [24]. Consistent with our study, no association has been detected between the MMP-7 A-181G polymorphism and the risk of hepatocellular carcinoma in a Chinese population [1]. A number of studies have reported on the association between the MMP-7 polymorphism with the risk of breast cancer with inconsistent results. Beeghly-Fadiel et al. [25] reported no significant association between the MMP-7 A-181G polymorphism and the risk of breast cancer among a Chinese population; however, they observed an increased risk of breast cancer among premenopausal women. The authors suggested that the common MMP-7 gene polymorphism could be a significant determinant of survival among Chinese women with breast cancer. Also, Hughes et al. [2] reported that there was a trend toward poor overall survival in breast cancer patients who were carriers of the MMP-7 GG genotype.
MMP-7 is a small metalloproteinase that is primarily expressed in breast epithelium. MMP-7 can degrade both ECM and non-ECM components from the cell surface [25]. Although MMP-7 is normally expressed in breast tissue, high expression of this protease leads to higher occurrences of mammary hyperplasia and accelerated tumor development. A number of studies have reported an association between MMP-7 variants and the risk of certain cancers [12,23,24,25]. However, in our study, we did not detect a statistically significant association between MMP7 A-181G and the risk of breast cancer among our population. This disparity might be attributable to differences in genetic background, the influence of environmental factors, gene-gene and gene-environment interactions, and the small sample size.
In conclusion, our findings indicate that the MMP-7 A-181G polymorphism may not be correlated with susceptibility to breast cancer in our population. We found a trend toward a higher percentage of lymph node metastasis, invasive lobular carcinoma, and ER-positive status in the presence of the combined genotype of MMP-7 AG+GG. Our findings could be confirmed and extended based on a larger sample in future.
Disclosure Statement
The authors declare no conflicts of interest.
Acknowledgment
This work was performed in partial fulfillment of the requirements for PhD by Research by Mr. KheirolahYari, Kermanshah University of Medical Sciences, Kermanshah, Iran, and was financially supported by a grant from Kermanshah University of Medical Sciences Office of the Vice Chancellor for Research, Kermanshah, Iran.
References
- 1.Qiu W, Zhou G, Zhai Y, Zhang X, Xie W, Zhang H, Yang H, Zhi L, Yuan X, Zhang X, He F. No association of MMP-7, MMP-8, and MMP-21 polymorphisms with the risk of hepatocellular carcinoma in a Chinese population. Cancer Epidemiol Biomarkers Prev. 2008;17:2514–2518. doi: 10.1158/1055-9965.EPI-08-0557. [DOI] [PubMed] [Google Scholar]
- 2.Hughes S, Agbaje O, Bowen RL, Holliday DL, Shaw JA, Duffy S, Jones JL. Matrix metalloproteinase single-nucleotide polymorphisms and haplotypes predict breast cancer progression. Clin Cancer Res. 2007;13:6673–6680. doi: 10.1158/1078-0432.CCR-07-0884. [DOI] [PubMed] [Google Scholar]
- 3.Kotepui M, Thawornkuno C, Chavalitshewinkoon-Petmitr P, Punyarit P, Petmitr S. Quantitative real-time RT-PCR of ITGA7, SVEP1, TNS1, LPHN3, SEMA3G, KLB and MMP13 mRNA expression in breast cancer. Asian Pac J Cancer Prev. 2012;13:5879–5882. doi: 10.7314/apjcp.2012.13.11.5879. [DOI] [PubMed] [Google Scholar]
- 4.Motie MR, Besharat S, Torkjazi R, Shojaa M, Besharat M, Keshtkar A, Roshandel G, Besharat S, Fateme AA. Modifiable risk of breast cancer in Northeast Iran: hope for the future. A case-control study. Breast Care (Basel) 2011;6:453–456. doi: 10.1159/000335203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boroujeni HR, Karimi M, Moshkelani S, Parsaei P. Association of the p53 codon 72 polymorphism with breast cancer in central part of Iran. Afr J Pharm Pharmacol. 2013;7:356–359. [Google Scholar]
- 6.González-Arriaga P, Pascual T, García-Alvarez A, Fernández-Somoano A, López-Cima MF, Tardón A. Genetic polymorphisms in MMP 2, 9 and 3 genes modify lung cancer risk and survival. BMC Cancer. 2012;12:121. doi: 10.1186/1471-2407-12-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahimi Z, Rahimi Z, Shahsavandi MO, Bidoki K, Rezaei M. MMP-9 (-1562 C: T) polymorphism as a biomarker of susceptibility to severe pre-eclampsia. Biomark Med. 2013;7:93–98. doi: 10.2217/bmm.12.95. [DOI] [PubMed] [Google Scholar]
- 8.Rahimi Z, Rahimi Z, Aghaei A, Vaisi-Raygani A. AT2R −1332 G:A polymorphism and its interaction with AT1R 1166 A:C, ACE I/D and MMP-9 −1562 C:T polymorphisms: risk factors for susceptibility to preeclampsia. Gene. 2014;538:176–181. doi: 10.1016/j.gene.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Zhou P, Du LF, Lv GQ, Yu XM, Gu YL, Li JP, Zhang C. Current evidence on the relationship between four polymorphisms in the matrix metalloproteinases (MMP) gene and breast cancer risk: a meta-analysis. Breast Cancer Res Treat. 2011;127:813–818. doi: 10.1007/s10549-010-1294-0. [DOI] [PubMed] [Google Scholar]
- 10.Srivastava P, Pandey S, Mittal B, Mittal RD. No association of matrix metalloproteinase (MMP)-2 (-735C>T) and tissue inhibitor of metalloproteinase (TIMP)-2 (-418G>C) gene polymorphisms with cervical cancer susceptibility. Indian J Clin Biochem. 2013;28:13–18. doi: 10.1007/s12291-012-0237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beeghly-Fadiel A, Long JR, Gao YT, Li C, Qu S, Cai Q, Zheng Y, Ruan ZX, Levy SE, Deming SL, Snoddy JR, Shu XO, Lu W, Zheng W. Common MMP-7 polymorphisms and breast cancer susceptibility: a multistage study of association and functionality. Cancer Res. 2008;68:6453–6459. doi: 10.1158/0008-5472.CAN-08-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Jin X, Fang S, Wang R, Li Y, Wang N, Guo W, Wang Y, Wen D, Wei L, Dong Z, Kuang G. The functional polymorphism in the matrix metalloproteinase-7 promoter increases susceptibility to esophageal squamous cell carcinoma, gastric cardiac adenocarcinoma and non-small cell lung carcinoma. Carcinogenesis. 2005;26:1748–1753. doi: 10.1093/carcin/bgi144. [DOI] [PubMed] [Google Scholar]
- 13.Saeed HM, Alanazi MS, Parine NR, Shaik J, Semlali A, Alharbi O, Azzam N, Aljebreen A, Almadi M, Shalaby MA. Matrix metalloproteinase-2 (-1306 C>T) promoter polymorphism and risk of colorectal cancer in the Saudi population. Asian Pac J Cancer Prev. 2013;14:6025–6030. doi: 10.7314/apjcp.2013.14.10.6025. [DOI] [PubMed] [Google Scholar]
- 14.Srivastava P, Kapoor R, Mittal RD. Association of single nucleotide polymorphisms in promoter of matrix metalloproteinase-2, 8 genes with bladder cancer risk in Northern India. Urol Oncol. 2013;31:247–254. doi: 10.1016/j.urolonc.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Rahimi Z, Merat A, Gerard N, Krishnamoorthy R, Nagel RL. Implications of the genetic epidemiology of globin haplotypes linked to the sickle cell gene in southern Iran. Hum Biol. 2006;78:719–731. doi: 10.1353/hub.2007.0016. [DOI] [PubMed] [Google Scholar]
- 16.Rahimi M, Hasanvand A, Rahimi Z, Vaisi-Raygani A, Mozafari H, Rezaei M, Zargooshi J, Najafi F, Shakiba E. Synergistic effects of the MTHFR C677T and A1298C polymorphisms on the increased risk of micro-and macro-albuminuria and progression of diabetic nephropathy among Iranians with type 2 diabetes mellitus. Clin Biochem. 2010;43:1333–1339. doi: 10.1016/j.clinbiochem.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Jormsjö S, Whatling C, Walter DH, Zeiher AM, Hamsten A, Eriksson P. Allele-specific regulation of matrix metalloproteinase-7 promoter activity is associated with coronary artery luminal dimensions among hypercholesterolemic patients. Arterioscler Throm Vasc Biol. 2001;21:1834–1839. doi: 10.1161/hq1101.098229. [DOI] [PubMed] [Google Scholar]
- 18.Taghavi A, Fazeli Z, Vahedi M, Baghestani AR, Pourhoseingholi A, Barzegar F, Pourhoseingholi MA. Increased trend of breast cancer mortality in Iran. Asian Pac J Cancer Prev. 2012;13:367–370. doi: 10.7314/apjcp.2012.13.1.367. [DOI] [PubMed] [Google Scholar]
- 19.Wang F, Jin XP, Zhu M, Lin XF, Hu XF, Wang WF, Han Z, Huang LZ. Genotype association of C(-735)T polymorphism of the MMP-2 gene with the risk of carotid atherosclerosis-vulnerable plaque in the Han Chinese population. Vasc Med. 2011;16:13–18. doi: 10.1177/1358863X10394237. [DOI] [PubMed] [Google Scholar]
- 20.Hajihoseini S, Bahmani M, Khosravi A, Ghezelsofla E, Ghaderi A. Prognostic significance of MMP2 and MMP9 functional promoter single nucleotide polymorphisms in head and neck squamous cell carcinoma. Iran J Basic Med Sci. 2011;14:137–144. [Google Scholar]
- 21.Sharma KL, Misra S, Kumar A, Mittal B. Higher risk of matrix metalloproteinase (MMP‐2, 7, 9) and tissue inhibitor of metalloproteinase (TIMP‐2) genetic variants to gallbladder cancer. Liver Int. 2012;32:1278–1286. doi: 10.1111/j.1478-3231.2012.02822.x. [DOI] [PubMed] [Google Scholar]
- 22.Kesh K, Subramanian L, Ghosh N, Gupta V, Gupta A, Bhattacharya S, Mahapatra NR, Swarnakar S. Association of MMP7 −181A/G promoter polymorphism with gastric cancer risk influence of nicotine in differential allele-specific transcription via increased phosphorylation of cAMP-response element-binding protein (CREB) J Biol Chem. 2015;290:14391–14406. doi: 10.1074/jbc.M114.630129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghilardi G, Biondi ML, Erario M, Guagnellini E, Scorza R. Colorectal carcinoma susceptibility and metastases are associated with matrix metalloproteinase-7 promoter polymorphisms. Clin Chem. 2003;49:1940–1942. doi: 10.1373/clinchem.2003.018911. [DOI] [PubMed] [Google Scholar]
- 24.Kubben F, Sier C, Meijer M, Van Den Berg M, Van Der Reijden J, Griffioen G, Van De Velde C, Lamers C, Verspaget H. Clinical impact of MMP and TIMP gene polymorphisms in gastric cancer. Br J Cancer. 2006;95:744–751. doi: 10.1038/sj.bjc.6603307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beeghly‐Fadiel A, Shu Xo, Long J, Li C, Cai Q, Cai H, Gao YT, Zheng W. Genetic polymorphisms in the MMP‐7 gene and breast cancer survival. Int J Cancer. 2009;124:208–214. doi: 10.1002/ijc.23859. [DOI] [PMC free article] [PubMed] [Google Scholar]


