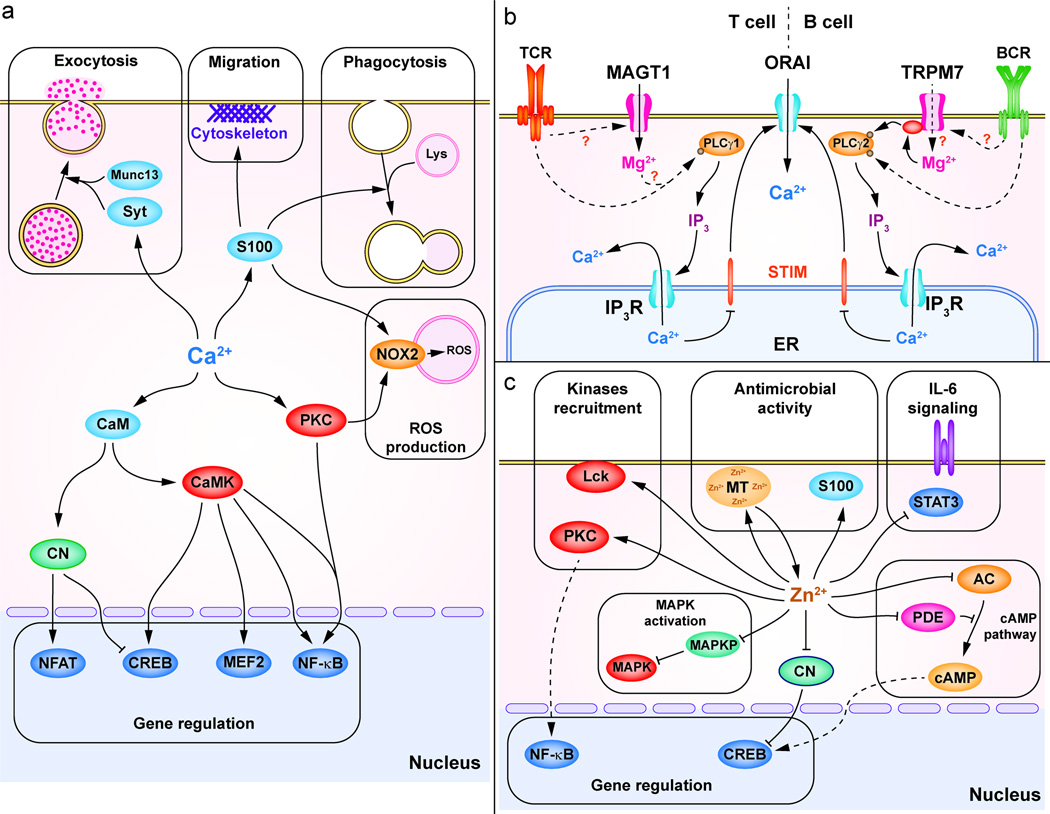
Figure 2. Divalent cations effectors and functions in immune cells.
(a) A broad range of functions can be rapidly deployed through Ca2+ signals. One major Ca2+ function is the regulation of gene expression involved in cell activation, differentiation and cytokine production. Ca2+ controls the activation of NFAT via CaM-CN, CREB and myocyte enhancer factor 2 (MEF2) via CaMK and CaM and NF-κB via PKC and CaMKII. Ca2+ controls exocytosis through Ca2+-sensitive proteins such as synaptotagmins (Syt) and Munc13. S100 proteins are Ca2+-binding proteins involved in the cytoskeleton organization during migration, lysosome-phagosome fusion during phagocytosis. In neutrophils, Ca2+ controls ROS production by NOX2 via PKC and S100 proteins (S100A8/A9). (b) In T and B cells, Mg2+ is regulating Ca2+ mobilization via SOCE, by modulating PLC-γ activation. In T cells, the TCR-induced MAGT1-dependent Mg2+ flux is required to activate PLC-γ1. In B cell, TRPM7 kinase domain participates in PLC-γ2 activation together with Bruton’s tyrosine kinase (BTK) in a Mg2+-dependent manner. (c) Zn2+ is involved in the recruitment of kinases such as PKC or LCK. Zn2+ enhances MAPK activation through inhibition of the MAPKP. Zn2+ controls gene expression through NF-κB and through inhibition of CN (which promotes CREB activation). In innate immune cells, Zn2+ binding-proteins such as MT and S100 proteins (S100A8/A9) are playing anti-microbial roles by chelating Zn2+. Zn2+ inhibits IL-6 signaling by inhibiting the activation of STAT3. Finally, Zn2+ regulates the cAMP pathway through the inhibition of AC or PDE.