Abstract
Purpose
Continuous transversus abdominis plane (TAP) block using a catheter has proven its usefulness in reducing opioid requirements and pain scores after lower abdominal surgery. However, there are no reports of its successful use after renal transplant. We tested the hypothesis that continuous TAP block would retrospectively reduce opioid requirement, nausea score and hospital stay after renal transplant surgery.
Methods
In a retrospective study, we reviewed the data from 63 adult renal transplant recipients—31 with patient-controlled TAP analgesia with standing orders for intravenous as well as oral opioids as needed and 32 with intravenous patient-controlled analgesia. The TAP catheter was inserted preoperatively using an ultra-sound-guided technique. Infusion of ropivacaine 0.2 % at 8 ml basal, 12 ml bolus and a lockout interval of 60 min were maintained for 48 h postoperatively. The primary outcome was total morphine-equivalent dose during the 48-h postoperative period. Secondary outcomes were pain and nausea scores for the 48-h postoperative period.
Results
The mean 48-h postoperative morphine-equivalent doses [95 % confidence interval] for patient-controlled intravenous analgesia and TAP catheter were 197 [111, 349] and 50 [28, 90], respectively, which were significantly different (P = 0.002). The mean 48-h average verbal response pain scores were 2.94 [2.39, 3.50] and 2.49 [1.93, 3.06], respectively, which were not significantly different (P = 0.26). The mean nausea scores were 0.66 [0.46, 0.87] and 0.60 [0.40, 0.81], respectively, which were not significantly different (P = 0.69). There was no difference regarding hospital stay.
Conclusion
The use of continuous TAP analgesia for postoperative analgesia after renal transplant was effective in reducing the morphine-equivalent requirements.
Keywords: Local anesthetic, ropivacaine, Opioid analgesics, morphine, Abdominal surgery, Kidney transplantation, TAP catheter, Nausea
Introduction
Renal transplant (RT) is now considered the best therapeutic option for end-stage renal disease as it prolongs life as well as improving quality and quantity of life [1]. In the United States, 16,000 RTs are performed each year1 and effective postoperative pain management contributes to outcomes [2]. Currently, opioid intravenous patient-controlled analgesia (IVPCA) is considered the mainstay method for postoperative pain control after RT surgery [3].
The transversus abdominis plane (TAP) block was first described by Rafi [4] using the lumbar triangle of Petit as a landmark regional anesthesia technique that provides abdominal wall analgesia. Ultrasound-guided TAP block was later described by Hebbard et al. [5]. The use of a continuous TAP block catheter is an attractive method to provide postoperative analgesia after renal transplantation. The continuous TAP catheter block has the advantages of dermatomal coverage for classic renal transplant incision (T10-L1), is not contraindicated in patients with preoperative coagulopathy which precludes neuroaxial block, and is easy to perform in the preoperative period. However, the pain relief by the unilateral continuous TAP catheter is not sufficient for RTs performed with midline incision.
We present the first report of ultrasound-guided continuous TAP block for postoperative pain relief after RT surgery using a catheter in the plane between the internal oblique and transversus abdominis muscles. Specifically, we tested the primary hypothesis that the use of continuous TAP blockade is associated with decreased 48-h postoperative opioid consumption in RT recipients compared to a group of control patients receiving a traditional analgesic approach. We also compared mean 48-h postoperative pain scores and mean 48-h postoperative nausea scores.
Methods
In a retrospective IRB approved study, we collected perioperative information from 63 patients undergoing RT—31 with continuous TAP catheter analgesia and 32 with IVPCA. Exclusion criteria for continuous TAP catheter analgesia were midline incision, combined nephrectomy with transplant, and patient refusal. Most of the surgeries were performed by two surgeons. Control group patients did not have TAP catheters. All data were obtained electronically from our electronic medical records. Preoperatively, patients scheduled for RT via unilateral flank incision were consented to have TAP block and catheter placement. The TAP catheter was inserted utilizing an ultrasound-guided technique to identify the catheter's position in the lateral abdominal wall before surgery [2]. Linear ultrasound transducers (13-6 MHz Sonosite or 12-5 MHz GE) were used for all procedures. The ultrasound probe was positioned behind the anterior axillary line midway between costal margin and iliac crest.
A 17-gauge Tuohy needle was advanced with an in-plane technique until the plane between internal oblique and transversus abdominis muscles was reached. After identifying the needle tip was in the plane between the two muscles, 20 ml of ropivacaine 0.5 % was injected and a single-orifice catheter (Arrow peripheral nerve catheter) was advanced ~5–7 cm past the needle tip in the plane between the muscles. The catheter was then tunneled and secured to the patient's back. Continuous infusion through the catheter was started in the recovery room using ropivacaine 0.2 % at 8 ml basal, 12 ml bolus and a lockout interval of 60 min. The infusion was maintained for 3 days (until postoperative day [POD] 2) and was discontinued once the patient was able to tolerate oral medication. The patients in the TAP group were instructed to utilize their bolus dose as needed; in addition, there were standing orders for intravenous as well as oral opioids if the pain was not controlled. Patients who did not have a TAP block received IVPCA. The opioids commonly used for IVPCA after RT surgery at our institution are either hydromorphone or fentanyl. In hydromorphone IVPCA, the initial starting demand dose was 0.2 mg every 6–10 min and was titrated upwards in 0.1 mg increments as needed. Clinician doses (CD) were started at 0.2–0.4 mg every 2 h according to the patient's request and increased as the demand dose was increased. However, in fentanyl IVPCA the initial starting demand dose was 20 μg every 6 min and was titrated upwards in 10 μg increments as needed. The CD were started at 25–50 μg every 1–2 h per patient request and increased as demand dose was increased. Verbal response scale pain scores, nausea and vomiting scores and opioid consumption throughout the first 48-h postoperative period were recorded. Opioids were converted to intravenous morphine-equivalent dosages using conversion factors published elsewhere [6].
Statistical analysis
Patient age, body mass index, gender, and year/month of surgery were considered for adjustment in the analysis and summarized for each group using standard descriptive statistics, as appropriate. Standardized difference scores—defined as the difference in means, mean rankings, or proportions divided by a combined estimate of standard deviation—were used to quantify the degree of imbalance between groups. Any of these variables exhibiting a standardized difference score of >0.1 standard deviations was used for adjustment in all group comparisons on outcomes. However, we were unable to adjust for year of surgery as there was insufficient overlap in this factor between IVPCA and TAP catheter patients.
For the primary hypothesis (comparing postoperative intravenous [IV] morphine-equivalent doses), we utilized multivariable linear regression. The logarithmic transformation was applied to the patients’ doses prior to modeling in order to estimate the percent difference in geometric means. For the secondary outcomes (48-h mean visual analog scale [VAS] pain score and 48-h mean nausea score), we used multivariable linear regression to estimate differences in means. All tests were model-based Wald chi-squared tests and were performed at a significance level of 0.05.
Results
Baseline, intraoperative, and postoperative measures for the 63 patients are given in Table 1. Although only small meaningful differences were observed between groups with regard to age, gender, and body mass index, we adjusted for these three factors as the standardized difference scores were all slightly greater than our threshold of 0.1.
Table 1.
Summary of patient demographic variables and year of treatment by study group
| IVPCA (N = 32) | TAP catheter (N = 31) | ASD | |
|---|---|---|---|
| Age (years) (mean ± SD)* | 50 ± 11 | 52 ± 14 | 0.15 |
| Female gender (%)* | 32 | 23 | 0.20 |
| Body mass index (kg/m2) (median [Q1, Q3])* | 31 [24, 32] | 27 [24, 31] | 0.19 |
| Month/year of treatment (median [Q1, Q3]) | 10/2008 [10/2008, 04/2009] | 10/2009 [05/2009, 04/2010] | 2.41 |
| Living donor (%) | 48 | 87 | 0.90 |
| Duration of surgery (h) (mean ± SD) | 5:59 ± 1:47 | 7:01 ± 1:34 | 0.62 |
| Estimated blood loss (cc) (median [Q1, Q3]) | 350 [188, 500] | 310 [250, 423] | 0.03 |
| Intraoperative opioids (mg) (morphine Eq., median [Q1, Q3]) | 27 [18, 38] | 30 [24, 38] | 0.22 |
| Serum creatinine POD1 (mg/dl) (mean ± SD) | 5.7 ± 3.1 | 5.7 ± 2.8 | 0.02 |
| Serum creatinine POD2 (mg/dl) (mean ± SD) | 4.4 ± 3.0 | 4.2 ± 2.8 | 0.06 |
| Postoperative acetaminophen dose (mg) (%) | |||
| 0 mg | 81 | 80 | 0.21 |
| 650 mg | 13 | 13 | |
| 1,300 mg | 6 | 0 | |
| 1,950 mg | 0 | 3 | |
| 2,600 mg | 0 | 3 | |
| Postoperative ondansetron dose (mg) (%) | |||
| 0 mg | 77 | 83 | 0.88 |
| 4 mg | 13 | 7 | |
| 8 mg | 3 | 3 | |
| 12 mg | 6 | 7 | |
ASD Absolute standardized differences—defined as the absolute value of the difference in means, mean rankings, or proportions divided by a combined estimate of standard deviation—are also reported. Asterisks denote variables used for adjustment in the analysis
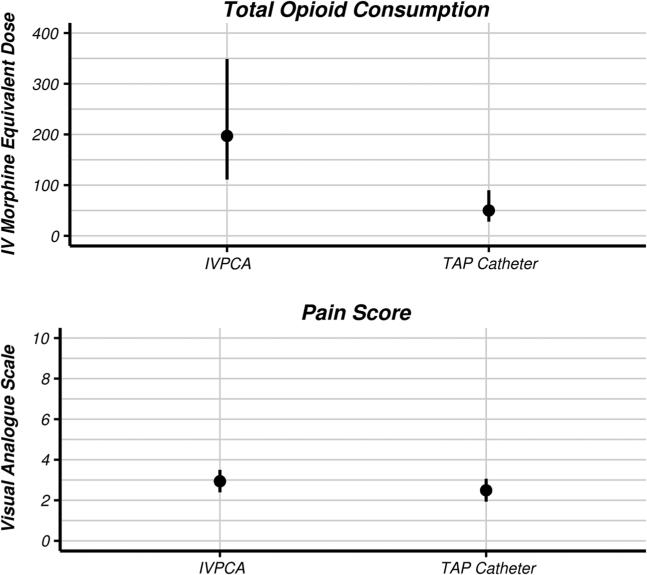
Based on our multivariable linear regression model, the adjusted geometric mean 48-h postoperative IV morphine-equivalent doses [95 % confidence interval] for the IVPCA and TAP catheter groups were 197 [111, 349] mg and 50 [28, 90] mg, respectively, corresponding to a ratio of means of 0.26 [0.11, 0.58], which was statistically different from a ratio of 1.0 (P = 0.002, Wald test).
The mean 48-h average VAS pain scores for the two groups were 2.94 [2.39, 3.50] and 2.49 [1.93, 3.06], respectively, corresponding to a difference in means of −0.45 [−1.24, 0.34] units, which was not statistically significant (P = 0.26). Twenty-two out of 30 of the TAP catheter patients had pain scores at ambulation in the dataset. Median [Q1, Q3] pain at ambulation for these patients was 6 [4, 7] on POD1 and 4 [3, 5] on POD2 (Fig. 1). The mean nausea scores were 0.66 [0.46, 0.87] and 0.60 [0.40, 0.81], respectively, corresponding to a difference in means of −0.06 [−0.35, 0.23], which was not statistically significant (P = 0.69).
Fig. 1.
Adjusted mean 48-h IV morphine-equivalent dose and adjusted mean 48-h visual analog pain scale for the IVPCA and TAP catheter groups. IV intravenous, IVPCA intravenous patient-controlled analgesia, TAP transversus abdominis plane
Discussion
This study extends continuous TAP analgesia for postoperative pain relief after RT surgery. Effective postoperative pain management continues to be an important outcome following RT. Uncontrolled pain may lead to agitation, tachycardia, hypertension and increased risks of respiratory complications [2]. Currently, the main stay for postoperative pain control after RT is opioid IVPCA. In the United Kingdom, 24 out of 27 RT centers use IVPCA for postoperative pain control [3]; however, only 2 of the 27 centers used epidural analgesia only in selected cases [5].
The use of opioids has many side-effects including impaired gastric motility with ileus, nausea, vomiting, and respiratory depression. Furthermore, opioids have adverse effects on the immune system which might increase the incidence of infection after RT [7]. Patients with renal failure typically have platelet dysfunction which some consider a relative contraindication for using epidural analgesia for postoperative pain control [8]. Regional nerve blocks like paravertebral and combined intercostal with ilioinguinal and iliohypogastric had been tried for postoperative pain control after RT. In spite of their applicability, none of these techniques use a catheter to achieve continuous analgesia.
TAP block is used successfully for pain relief for abdominal procedures including hernia repair, hysterectomy, cesarean delivery and suprapubic prostatectomy [9, 10]. Some practitioners also suggest its role in the differential diagnosis of chronic abdominal pain [11]. The use of ultrasound during the TAP block technique helps to determine catheter placement in the lateral abdominal wall avoiding the surgical field, and also makes the TAP block more accurate. Tran et al. [12] demonstrated in a cadaveric study that an ultrasound-guided TAP injection cephalad to the iliac crest is likely to involve the T10-L1 nerve roots, which makes TAP block very effective for classic RT incision. In addition, there is no intraperitoneal involvement which eliminates the visceral pain component. The efficacy of TAP block for pain control after RT is controversial. The first report of using TAP block for RT by Mukhtar et al. [13] showed decreased opioid requirements, pain scores, and nausea and vomiting in the postoperative period. However, a recent report by Freir et al. [14] did not show any beneficial effect from using TAP block for pain relief in RT. In both reports, the authors inserted a single-shot TAP block using the landmark technique. The use of the continuous TAP block technique in RT has been recently described [15]; however, the authors had to abandon the technique due to the low success rate (50 %) in catheter placement, low analgesic effect and surgical field interference. Instead, the authors asked the surgeons to insert the catheter under direct visualization. The authors did not describe in their report what technique they used in placing the TAP catheter or what kind of catheter they used [15].
Our study, however, indicated an association between the use of continuous TAP analgesia and reduced postoperative opioid requirements when compared to patients receiving IVPCA. The pain scores were to some extent lower with TAP analgesia but not statistically significant. One explanation for this can be attributed to the small number of patients in our study. Another explanation can be back pain induced by the patient's position or surgical manipulation during the procedure.
One patient in the TAP group had severe back pain unrelieved by systemic narcotics although the incisional area was numb due to the TAP catheter analgesia. An ultrasound scan showed a massive psoas hematoma which required surgical evacuation. If the patient had used IVPCA only, it may have masked the source of the back pain and delayed the diagnosis of psoas hematoma induced by surgery. We used ropivacaine for the local anesthetic infusion in our study due to its safety profile in patients with renal failure [16]. We kept the catheters in place for the 48-h postoperative period as keeping catheters >48 h would have increased the risk of local inflammation and infection in immunocompromised patients [17].
The main limitations of our study are the retrospective design and lack of documentation regarding pain score at cough or ambulation in patients with IVPCA, which allowed us only to show an association between the use of continuous TAP block catheter and postoperative opioid consumption in RT. Furthermore, we used historical control patients in our study, which may have introduced a bias.
Conclusions
We present the first use of the ultrasound-inserted continuous TAP block catheter technique for postoperative pain control after RT. Our preliminary results showed that this technique might be associated with reduced opioid consumption after renal transplantation. However, larger randomized trials are needed to confirm the results.
Acknowledgments
This work was supported by internal department funds.
Footnotes
U.S. Renal Data System, USRDS 2006 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2006. The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. government. Available from: http://www.usrds.org/atlas06.aspx (Accessed December 2012).
Conflict of interest The authors have nothing to disclose.
Contributor Information
Ehab Farag, Departments of General Anesthesiology and Outcomes Research, Cleveland Clinic, 9500 Euclid Avenue/E31, Cleveland, OH 44195, USA.
Maged N. Guirguis, Department of Pain Management, Cleveland Clinic, Cleveland, OH, USA
Mada Helou, Anesthesiology Institute, Cleveland Clinic, Cleveland, OH, USA.
Jarrod E. Dalton, Departments of Quantitative Health Sciences and Outcomes Research, Cleveland Clinic, Cleveland, OH, USA
Fallon Ngo, Anesthesiology Institute, Cleveland Clinic, Cleveland, OH, USA.
Michael Ghobrial, Anesthesiology Institute, Cleveland Clinic, Cleveland, OH, USA.
Jerome O'Hara, Department of General Anesthesiology, Cleveland Clinic, Cleveland, OH, USA.
John Seif, Department of Pediatric Anesthesiology, Cleveland Clinic, Cleveland, OH, USA.
Venkatesh Krishnamurthi, Department of Urology, Cleveland Clinic, Cleveland, OH, USA.
David Goldfarb, Department of Urology, Cleveland Clinic, Cleveland, OH, USA.
References
- 1.Zhang R, Kumar P, Ramcharan T, Reisin E. Kidney transplantation: the evolving challenges. Am J Med Sci. 2004;328:156–61. doi: 10.1097/00000441-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Jankovic Z, Sri-Chandana C. Anaesthesia for renal transplant: recent developments and recommendations. Curr Anaesth Crit Care. 2008;19:247–53. [Google Scholar]
- 3.Williams M, Milner QJ. Postoperative analgesia following renal transplantation––current practice in the UK. Anaesthesia. 2003;58:712–3. doi: 10.1046/j.1365-2044.2003.32661.x. [DOI] [PubMed] [Google Scholar]
- 4.Rafi AN. Abdominal field block: a new approach via the lumbar triangle. Anaesthesia. 2001;56:1024–6. doi: 10.1046/j.1365-2044.2001.02279-40.x. [DOI] [PubMed] [Google Scholar]
- 5.Hebbard P, Fujiwara Y, Shibata Y, Royse C. Ultrasound-guided transversus abdominis plane (TAP) block. Anaesth Intensive Care. 2007;35:616–7. [PubMed] [Google Scholar]
- 6.Turan A, Dalton JE, Keeyapaj W, Chu W, Bernstein E, Fu A, Jae Ho L, Saager L, Sessler DI. Preoperative angiotensin-converting enzyme inhibitor use is not associated with increased postoperative pain and opioid use. Clin J Pain. 2013;29:1050–6. doi: 10.1097/AJP.0b013e318287a258. [DOI] [PubMed] [Google Scholar]
- 7.Sanders RD, Hussell T, Maze M. Sedation and immunomodulation. Crit Care Clin. 2009;25:551–70. doi: 10.1016/j.ccc.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 8.United States Renal Data System . USRDS annual data report. USRDS Coordinating Center; Minneapolis, MN: 2006. [12/9/2012]. Available at: http://www.usrds.org/atlas.htm. [Google Scholar]
- 9.McDonnell JG, Curley G, Carney J, Benton A, Costello J, Maharaj CH, Laffey JG. The analgesic efficacy of transversus abdominis plane block after cesarean delivery: a randomized controlled trial. Anesth Analg. 2008;106:186–91. doi: 10.1213/01.ane.0000290294.64090.f3. [DOI] [PubMed] [Google Scholar]
- 10.O'Donnell BD, McDonnell JG, McShane AJ. The transversus abdominis plane (TAP) block in open retropubic prostatectomy. Reg Anesth Pain Med. 2006;31:91. doi: 10.1016/j.rapm.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Mournir Soliman L, Narouze S. Ultrasound-guided transversus abdominus plan block for the management of abdominal pain: an alternative to differential epidural block. Tech Reg Anesth Pain Manag. 2009;13:117–20. [Google Scholar]
- 12.Tran TM, Ivanusic JJ, Hebbard P, Barrington MJ. Determination of spread of injectate after ultrasound-guided transversus abdo-minis plane block: a cadaveric study. Br J Anaesth. 2009;102:123–7. doi: 10.1093/bja/aen344. [DOI] [PubMed] [Google Scholar]
- 13.Mukhtar K, Khattak I. Transversus abdominis plane block for renal transplant recipients. Br J Anaesth. 2010;104:663–4. doi: 10.1093/bja/aeq077. [DOI] [PubMed] [Google Scholar]
- 14.Freir NM, Murphy C, Mugawar M, Linnane A, Cunningham AJ. Transversus abdominis plane block for analgesia in renal transplantation: a randomized controlled trial. Anesth Analg. 2012;115:953–7. doi: 10.1213/ANE.0b013e3182642117. [DOI] [PubMed] [Google Scholar]
- 15.Jankovic ZB, Pollard SG, Nachiappan MM. Continuous trans-versus abdominis plane block for renal transplant recipients. Anesth Analg. 2009;109:1710–1. doi: 10.1213/ANE.0b013e3181ba75d1. [DOI] [PubMed] [Google Scholar]
- 16.Pere PJ, Ekstrand A, Salonen M, Honkanen E, Sjovall J, Henri-ksson J, Rosenberg PH. Pharmacokinetics of ropivacaine in patients with chronic renal failure. Br J Anaesth. 2011;106:512–21. doi: 10.1093/bja/aer002. [DOI] [PubMed] [Google Scholar]
- 17.Capdevila X, Bringuier S, Borgeat A. Infectious risk of continuous peripheral nerve blocks. Anesthesiology. 2009;110:182–8. doi: 10.1097/ALN.0b013e318190bd5b. [DOI] [PubMed] [Google Scholar]