Figure 2.
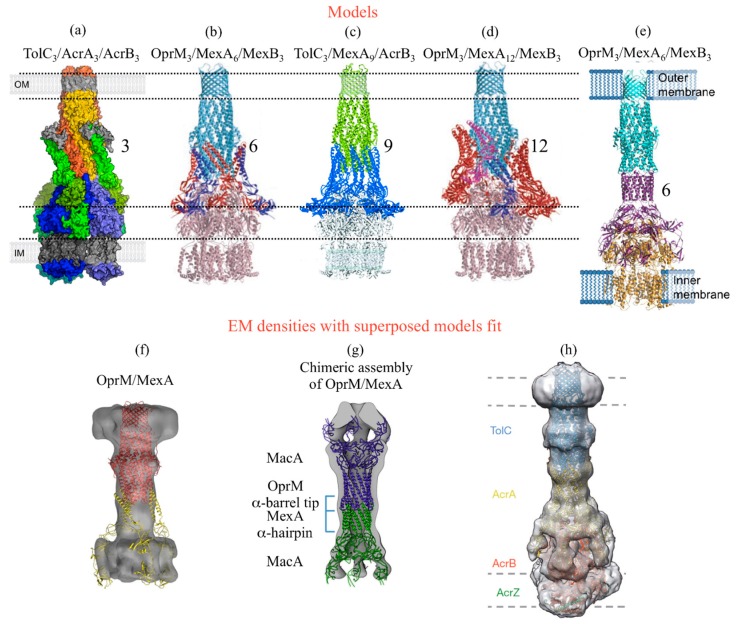
Published models and EM densities of the efflux pump assembly involving OprM or its E. coli counterpart TolC. (a) Surface representation of a proposed 3:3:3 assembly model for the TolC-AcrA-AcrB pump from E. coli coloured in orange, green and blue respectively (reproduced with permission from Symmons M.F. et al., Proceedings of the National Academy of Sciences [55]; published by The National Academy of Sciences, 2009). (b and d) Cartoon representations of the respectively proposed 3:6:3 and 3:12:3 assembly models for the MexA-MexB-OprM pump from P. aeruginosa. OprM and MexB are coloured light-blue and pink respectively. Monomers of MexA are in blue, red or magenta (reproduced with permission from Akama H. et al., Journal of Biological Chemistry [30]; published by The American Society for Biochemistry and Molecular Biology ©2004). (c) Cartoon representation of the proposed 3:9:3 assembly model of the chimeric TolC-MexA-AcrB pump, respectively coloured in green, blue and light-blue, based on the fit of their respective 3D structure. (reproduced with permission from Higgins M.K. et al., Proceedings of the National Academy of Sciences [31] “National Academy of Sciences, U.S.A ©2004”). (e) Cartoon representation of a proposed 3:6:3 assembly model for the OprM-MexA-MexB pump from P. aeruginosa, respectively coloured in cyan, purple and orange (reproduced with permission from Xu Y. et al., Journal of Biological Chemistry [70] published by The American Society for Biochemistry and Molecular Biology ©2011). (f) Electronic microscopy (EM) density for the P. aeruginosa OprM-MexA complex reconstituted into proteoliposomes, with a proposed 3:3 model equivalent to the one presented in (a) (reproduced with permission from Trépout S. et al., Biochem. Biophys. Acta [67]; published by Elsevier B.V. 2010). (g) EM density of a chimeric assembly of MacA (blue) in which the α-coiled-coil domains were replaced by those of OprM (purple) or MexA (yellow), together with the cartoon representation of the corresponding fitted model (reproduced with permission from Xu Y. et al., Journal of Biological Chemistry [71]; published by The American Society for Biochemistry and Molecular Biology ©2012). (h) EM density of a stabilised assembly of the E. coli TolC-AcrA-AcrB pump in complex with AcrZ together with the cartoon representation of the corresponding fitted model, respectively coloured in blue, yellow, red and green (reproduced with permission from Du D. et al., Nature [57]; published by Macmillan Publishers Ltd Copyright © 2014). The different models published before 2011 (a–d) were based on the hypothesis of a direct interaction between the RND and the OMF proteins, surrounded of variable oligomeric states of the MFP partner. On the contrary, all the EM densities are in favor of a tip-to-tip interaction between the MFP and the OMF. We can see in (f) the discrepancy between the two hypothesis, as the MexA structure positioned as in (a) does not fit with the OprM/MexA EM density.