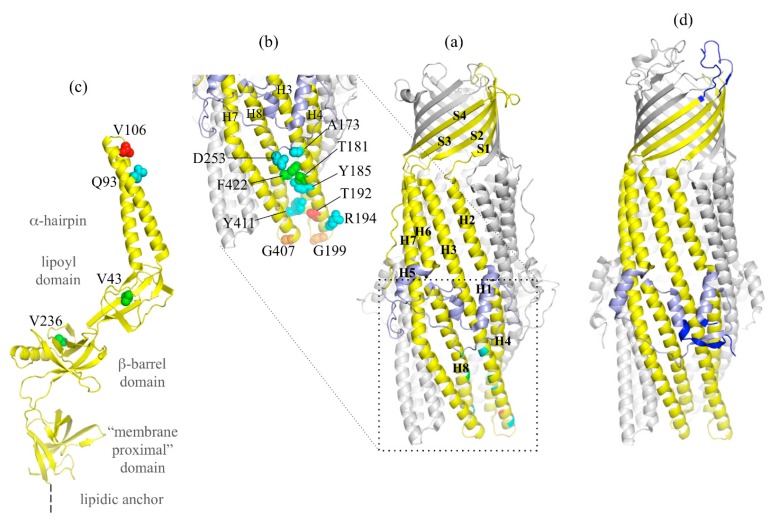
Figure 3.
OprM structure with reported gain of function mutants and α-coiled-coil domain mutations. (a) Cartoon representation of the OprM trimer with two monomers colored in grey and one monomer colored in yellow with the equatorial domain in light blue. The secondary structure adopted is indicated. (b) Zoom of the OprM α-coiled-coil domain. Residues colored in blue (A173, Y185, D253, Y411, and R194) correspond to the equivalent positions of the TolC mutants making TolC functional with MexAB. Residues colored in green (T181I and F422I) correspond to the OprM mutations suppressing the MexA-V106M loss of function. The residue colored in red (T192) corresponds to a loss of expression of OprM. Residues colored in orange (G199A and G407A) correspond to non-functional mutants. (c) Cartoon representation of one monomer of the MexA structure (PDB code 2V4D) [55] with the three residues cited in the text presented in sphere. V106, whose mutation in methionine leads to a non-functional MexAB-OprM pump, is in red. V43 and V236, whose mutation respectively in methionine and phenylalanine restore the loss of function of MexB-G220S, are in green. Q93, whose mutation in arginine makes MexA able to function with OprN, is in blue. (d) Cartoon representation of TolC for comparison with the same color code as in OprM, with the exception of the three regions largely different from OprM structure (N-terminus, extracellular loop and C-terminus), colored in blue.