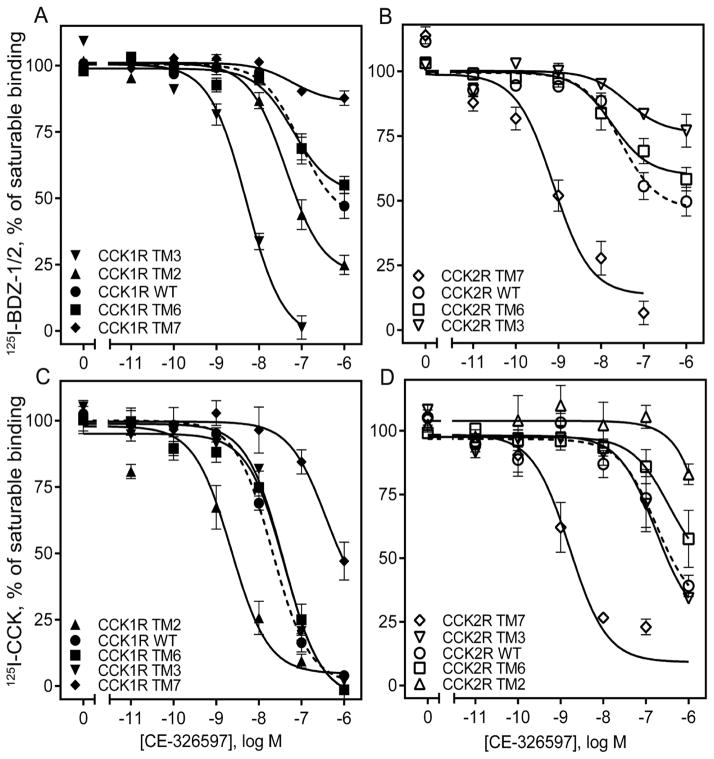
Figure 5. Competition-binding studies of CE-326597 using CCK1R- and CCK2R-based chimeric receptor constructs.
Shown are the competition-binding curves for CE-326597 at CCK1R (A, C) or CCK2R (B, D) based TM chimeric constructs using the allosteric antagonist radiolabels, 125I-BDZ-1 (for CCK1R) (A), 125I-BDZ-2 (for CCK2R) (B), or orthosteric CCK-like radiolabel (C, D). The X-axis values reflect the absence of ligand (0) to the left of the break, and log molar concentrations to the right of the break. Values represent percentages of maximal saturable binding that were observed in the absence of competitor. Non-saturable binding was determined by using 1 μM unlabeled BDZ-1 or BDZ-2, as appropriate for the radioligand, or CCK. Data are expressed as means ± S.E.M. of duplicate determinations from four to six independent experiments.