History
In February 2012, 12 transgenic zebrafish with an AB background were imported into the quarantine room at the Zebrafish International Resource Center (ZIRC; a part of the University of Oregon, Eugene, Ore), which is a repository and distribution center for thousands of mutant, transgenic, and wild-type zebrafish (Danio rerio) lines used by the research community. The imported fish were housed in 1-gallon aquaria on a flow-through water system with a UV sterilizer. Effluent from all tanks in the quarantine room drains to a sump. The sump contains only system effluent. Water is pumped from the sump to a 5-gallon sentinel tank. The sentinel tank is populated with 25 AB wild-type zebrafish, with no underlying health issues, from the ZIRC main fish facility. Effluent from the sentinel tank goes back to the same sump. Sump overflow drains to the municipal sewer. In the 3.5 months following arrival of the transgenic fish, high mortality rates were noticed in AB fish spawned with the transgenic line and in the AB quarantine room sentinel fish.
Clinical and Gross Findings
The 12 imported zebrafish carried a transgene encoding Cre recombinase expressed in pancreatic beta cells on an AB background. The Cre recombinase transgene alone does not result in a morphological phenotype (this line is typically crossed to another transgenic line containing loxP sites to induce Cre-mediated recombination and expression of the loxP-flanked target sequence in pancreatic beta cells in the progeny). On arrival, the fish were 233 days old and no physical or behavioral abnormalities were observed. That same month, when abnormally low pH was measured in the system water, 5 AB wild-type sentinel zebrafish from the quarantine room were euthanized by rapid chilling (hypothermic shock) via immersion in ice-chilled water for 10 minutes and underwent fixation for histologic evaluation. Histopathologic findings in the sentinel fish included infections with Pseudoloma neurophilia (microsporidiosis) and gill epithelial hyperplasia and hypertrophy. The gill changes are typically associated with suboptimal water quality. The remaining 20 sentinel fish were left in the tank.
In mid-March, the transgenic fish and AB wild-type fish from the ZIRC main fish facility, with no underlying health issues, were spawned in 4 small group crosses (3 transgenic with 3 AB). Transgenic and AB fish were together in a spawning tank with static water for 24 hours then moved to a new spawning tank with fresh, static water for an additional 24 hours. Spawning tanks were filled with system water from the quarantine room. Following spawning, the AB fish were also maintained on the flow-through water system in the quarantine room, in their own tank. Two weeks later, the AB outcross fish were underweight with multifocal hyper-pigmented epidermis. One AB fish died. The other 11 were euthanized by rapid chilling. At the same time, sentinel fish in the quarantine room began to appear underweight (Figure 1). Sentinel fish behavior would change from normal to rapid operculum movements near the drain, at the tank surface, or on the bottom of the tank. When this abnormal behavior was observed, approximately one-third of the sentinel tank water was drained and replaced with fresh system water. However, once behavior changed, fish died within 24 hours. Over 2 months, approximately 13 quarantine room sentinel fish were found dead or missing.
Figure 1.
Photograph of an emaciated zebrafish from a group of 25 AB wild-type zebrafish maintained in a 5-gallon sentinel tank into which effluent from all quarantine tanks at the Zebrafish International Resource Center (ZIRC) was deposited. Three months earlier, 12 transgenic zebrafish with an AB background were imported into the quarantine room. This fish is 2.0 cm (standard length).
In April, an emaciated fish was removed from the transgenic zebrafish tank, euthanized by rapid chilling, and fixed in Dietrich’s fixative.1 The other 11 transgenic zebrafish appeared physically and behaviorally normal. In May, the remaining 7 fish in the sentinel tank were similarly euthanized and fixed.
Histopathologic and Laboratory Findings
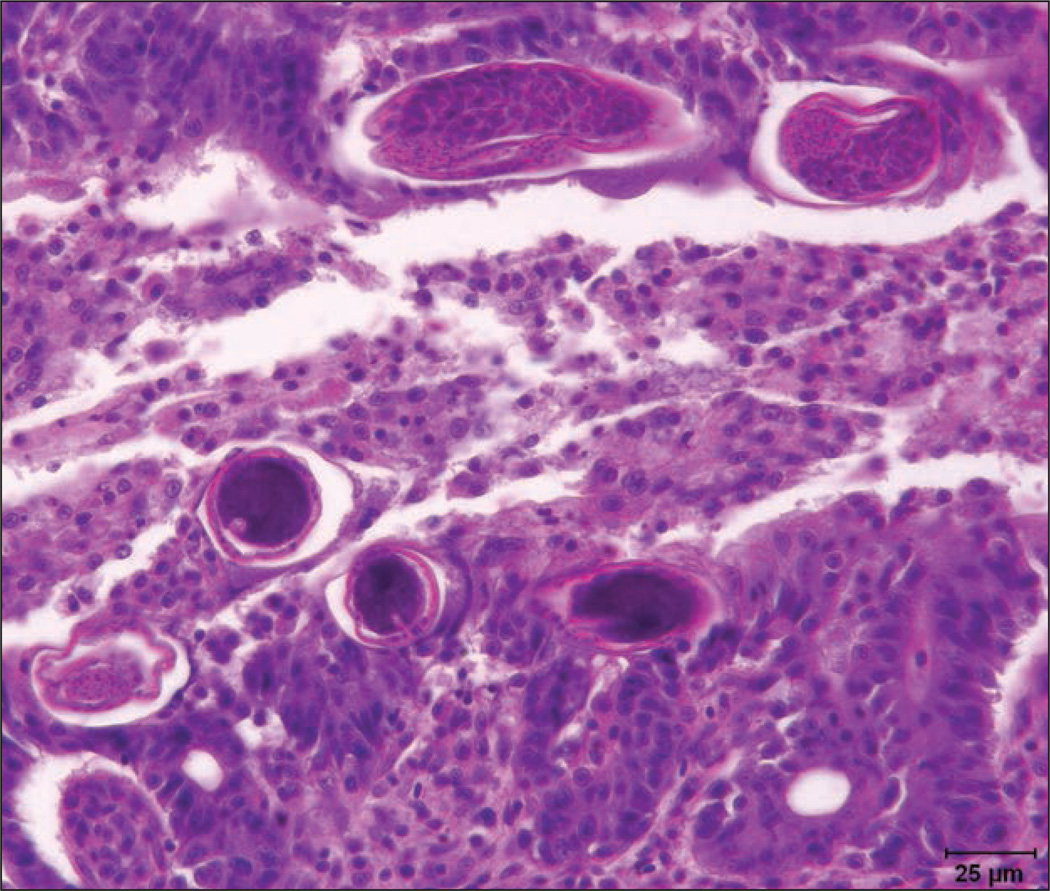
Fixed tissues from transgenic and sentinel zebrafish were routinely processed for preparation of H&E-stained paraffin sections. Collectively, among the fish examined, there was segmental to diffuse, variably severe, chronic and ongoing proliferative enteritis involving the proximal to distal portions of the intestinal tract, with the mid to distal aspect of the intestines most severely affected. Within affected regions of the intestines, the mucosal epithelium was markedly hyperplastic, had irregular folds, and had frequent fusion of rugae (rugae correspond to mammalian villi) with epithelial dysplasia. Mucosal epithelial cells were 3 to 6 layers deep and haphazardly piled up with loss of nuclear polarity. The cells displayed intense nuclear basophilia with granular marginated chromatin, 2 to 4 prominent nucleoli, nuclear molding, and frequent karyomegaly. Among the dysplastic epithelial cells, there was mild anisocytosis and increased mitoses, which often had aberrant sun-burstlike or, rarely, tripolar configurations. The underlying lamina propria was edematous and expanded with dense infiltrates of chronic inflammatory cells, which often percolated throughout the epithelium. Infiltrates were primarily composed of intermixed lymphocytes, eosinophilic granule cells, neutrophils, and fewer histiocytes. Burrowed into the mucosal epithelium and subepithelial spaces of rugae as well as penetrating deep between rugal folds were variable numbers of male and female capillarid nematodes in longitudinal and transverse sections, measuring approximately 16 to 40 µm in multiple transverse cross sections (Figure 2). The nematodes had morphological features consistent with Pseudocapillaria tomentosa, including an alternating amphophilic to basophilic periesophageal stichosome in females, hypodermal bacillary bands, and rarely, in gravid females, intrauterine larvated eggs that were barrel or lemon shaped with symmetric bipolar plugs. Eggs were approximately 40 to 65 µm in length. In some intestinal sections, several of these capillarid eggs were free within the lumen. Numerous intraproprial venules were ectatic with attenuated endothelium and individual venules were surrounded by perivenular cuffs of chronic inflammatory cell aggregates and extravasated proteinaceous fluid. In some of the venules, there was pavementing of neutrophils and lymphocytes along the endothelium. Neural microsporidiosis and gill epithelial hyperplasia and hypertrophy were also observed, consistent with findings in fish from the same sentinel tank population that were examined in February.
Figure 2.
Photomicrograph of a section of intestine from a euthanized AB wild-type zebrafish illustrating chronic ongoing proliferative enteritis and intralesional subepithelial Pseudocapillaria tomentosa nematodes in transverse and partial longitudinal sections. H&E stain; bar = 25 µm.
Debris was collected from the transgenic zebrafish tank by scrubbing all tank surfaces, allowing debris to settle, pouring off most of the water, and collecting the last 30 to 40 mL of water containing resuspended debris. The debris was processed for double centrifugation with saturated sugar solution as previously described. 2 The sample was split between two 15-mL tubes.a In half of the debris, 241 eggs were observed. Eggs were barrel-shaped, were larvated, had symmetric bipolar plugs, and were uniform in size and shape. One representative egg measured 32 × 65 µm (Figure 3).
Figure 3.
Photomicrograph of a parasitic egg found in debris of the tank in which the transgenic zebrafish had been maintained. The egg is larvated with symmetric bipolar plugs. Bar = 20 µm.
Morphologic Diagnosis and Case Summary
Morphologic diagnosis: severe, chronic and ongoing, segmental to diffuse, proliferative enteritis with epithelial dysplasia and intralesional nematodes consistent with P tomentosa.
Case summary: P tomentosa infection in zebrafish.
Comments
The weight loss in a single transgenic fish and morbidity and death in the AB sentinel fish was attributed to infections with the intestinal nematode P tomentosa. Clinical signs and progression in the AB outcross fish were also consistent with P tomentosa infection. Although some capillarids may go through an intermediate host before infecting fish, P tomentosa can be directly transmitted between zebrafish.3,4 Zebrafish with an AB background were exposed to the infected eggs while commingled with the transgenic fish for spawning and in the effluent from the transgenic tank flowing into the sentinel tank. Clinical disease and death in the AB fish occurred as soon as 2 weeks after exposure. All exposed AB fish eventually developed clinical signs. They died or were euthanized, and intestinal nematodes were subsequently identified in histologic sections of all these fish. The sentinel fish may have been compromised by the underlying gill lesions and microsporidiosis, conditions that were preexisting when the transgenic fish arrived. The gill lesions likely contributed to behavioral changes including rapid opercular beating, thereby increasing respiratory effort and ventilation, and moving toward the water surface (piping) in proximity to the water inflow. No changes in the renal hematopoeitic tissue were observed, indicating that this behavior was not a result of anemia. Although microsporidiosis has been associated with underweight fish,5 it is typically a chronic progression and does not result in acute death. The AB outcross fish had no underlying disease.
Interestingly, in contrast to findings for the AB fish, weight loss was observed in only one of the transgenic fish, even after the stress of shipping and introduction to a new water system. The high number of eggs identified in the tank debris and constant commingling suggested continuous exposure and the likelihood that others were infected. The transgenic fish may have enhanced immunity to the parasite or slower progression of disease and tissue invasion or may have reached some level of homeostasis with the parasite. Alternatively, the nematodes may have been host-adapted to the transgenic line. The subclinical nature of the infection in the transgenic fish on arrival highlights the importance of maintaining all new fish arrivals in a quarantine room and introducing only their progeny, as surface-sanitized embryos, into the main fish facility.
The possibility of early horizontal transmission of the parasite to the progeny should be considered. Pseudo-capillaria tomentosa eggs could potentially be shed with gametes, and the effect of bleach solutions (25 to 50 µL/ mL) typically used to surface-sanitize zebrafish embryos on P tomentosa eggs is not known. The sugar floatation and double centrifugation of tank debris is an attractive nonlethal alternative to histologic evaluation for diagnosis of capillariasis. It can be used for routine and repeated monitoring of whole tank populations. At the ZIRC, a combination of histologic evaluation and sugar floatation of tank debris was used to monitor the progeny of the transgenic and AB cross. No evidence of capillariasis was detected, and the line has since been retired.
The ZIRC Pathology Service has diagnosed P tomentosa infections in zebrafish from research facilities in the United States, Europe, Australia, and Japan. The sharing of zebrafish lines between facilities, lack of quarantine for transferred fish, direct life cycle of the parasite, and potential for nematode eggs to survive surface-sanitizing procedures may contribute to the spread of P tomentosa. Infections are associated with emaciation, hyperpigmentation, and death. Histologic lesions range from chronic enteritis to intestinal neoplasms.4 Pseudocapillaria tomentosa has been detected in a wide range of hosts, including 25 cyprinids, European eels, cod, salmon, and catfish.6,7 Among popular aquarium fishes, the nematode has been recovered from the intestines of goldfish, guppies (experimental host), and tiger barbs, most likely resulting in death in captive tiger barbs.6,8,9 Conspecific larvae were also identified in the liver of neon tetra.8 Noga10 lists fenbendazole and levamisole as potential treatments for nonencysted nematode infections in fish. In our experience, P tomentosa is frequently found within tissues in the intestinal wall of infected zebrafish. Recent publications describe emamectin benzoate, ivermectin, and fenbendazole treatments for capillariasis in zebrafish.11,12
Acknowledgments
The Zebrafish International Resource Center is supported by the National Institutes of Health Office of Research Infrastructure Programs (award P40OD011021).
The authors thank Dr. Michael Kent for technical advice.
Footnotes
BD Falcon Tubes, Fisher Scientific, Fremont, Calif.
References
- 1.Fournie JW, Krol RM, Hawkins WE. Fixation of fish tissues. In: Ostrander GK, editor. The laboratory fish. San Diego: Academic Press; 2000. pp. 569–578. [Google Scholar]
- 2.Foreyt WJ. Diagnostic parasitology. Vet Clin North Am Small Anim Pract. 1989;19:979–1000. doi: 10.1016/s0195-5616(89)50107-4. [DOI] [PubMed] [Google Scholar]
- 3.Moravec F, Prokopic J, Shlikas AV. The biology of nematodes of the family Capillariidae Neveu-Lemaire, 1936. Folia Parasitol (Praha) 1987;34:39–56. [PubMed] [Google Scholar]
- 4.Kent ML, Bishop-Stewart JK, Matthews JL, et al. Pseudocapillaria tomentosa, a nematode pathogen, and associated neoplasms of zebrafish (Danio rerio) kept in research colonies. Comp Med. 2002;52:354–358. [PubMed] [Google Scholar]
- 5.Matthews JL, Brown AM, Larison K, et al. Pseudoloma neurophilia n.g., n. sp., a new microsporidium from the central nervous system of the zebrafish (Danio rerio) J Eukaryot Microbiol. 2001;48:227–233. doi: 10.1111/j.1550-7408.2001.tb00307.x. [DOI] [PubMed] [Google Scholar]
- 6.Moravec F. Revision of capillarid nematodes (subfamily Capillariinae) parastitic in fishes. Prague: Academia natkadatelství Ceskoslovenské akademie ved; 1987. p. 273. [Google Scholar]
- 7.Aguilar A, Alvarez MF, Leiro JM, et al. Parasite populations of the European eel (Anguilla anguilla L.) in the rivers Ulla and Tea (Galicia, northwest Spain) Aquaculture. 2005;249:85–94. [Google Scholar]
- 8.Moravec F, Ergens R, Repova R. First record of the nematode Pseudocapillaria brevispicula(Linstow, 1873) from aquarium fishes. Folia Parasitol (Praha) 1984;31:241–245. [Google Scholar]
- 9.Lomakin VV, Trofimenko VYA. Capillariids (Nematoda: Capillariidae) of the freshwater fish fauna of the USSR. Trudi Gelmintologicheskogo Inst Akad Nauk. 1982;31:60–87. [Google Scholar]
- 10.Noga EJ. Fish disease. Ames, Iowa: Iowa State University Press; 2000. pp. 286–288. [Google Scholar]
- 11.Collymore C, Watral V, White JR, et al. Tolerance and efficacy of emamectin benzoate and ivermectin for the treatment of Pseudocapillaria tomentosa in laboratory zebrafish (Danio rerio) Zebrafish. 2014;11:490–497. doi: 10.1089/zeb.2014.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maley D, Laird AS, Rinkwitz S, et al. A simple and efficient protocol for the treatment of zebrafish colonies infected with parasitic nematodes. Zebrafish. 2013;10:447–450. doi: 10.1089/zeb.2013.0868. [DOI] [PubMed] [Google Scholar]