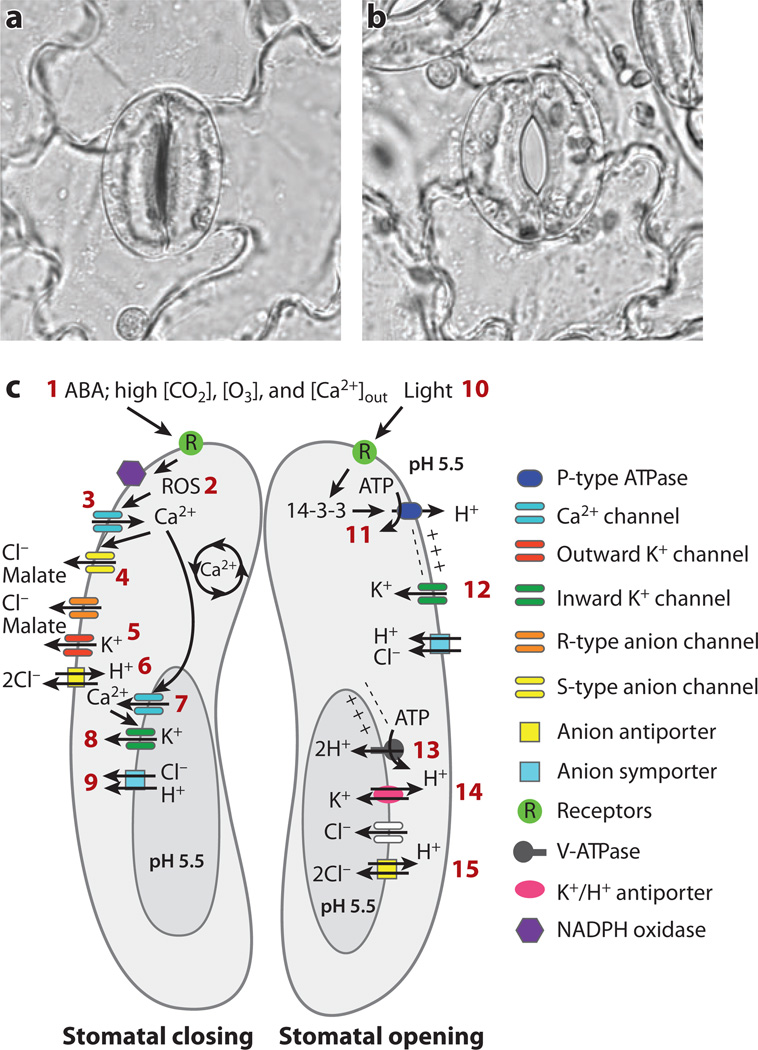
Figure 1.
Model for ion channel and transporter classes and their demonstrated or predicted roles during stomatal movements. (a) A closed stomate in a leaf from Vicia faba (broad bean). (b) An open stomate from Vicia faba. Two guard cells surround the stomatal pore and regulate the aperture of the central pore. (c) Model for regulation and activity of guard cell ion channels and transporters. (Left) (1) Signals that induce stomatal closing include the hormone abscisic acid (ABA), high [CO2], high extracellular [Ca2+], and high ozone [O3]. Many of the receptors involved remain to be identified (see Reference 26). Stomatal closing requires net cellular efflux of solutes, in particular K+, Cl, and malate. ABA induces reactive oxygen species (ROS) production (2), which activates Ca2+-permeable ICa channels (3). Cytosolic Ca2+ concentration ([Ca2+]cyt) is a central regulator of transport mechanisms in guard cells and activates slow/sustained (S-type) anion channels (4) and vacuolar SV (TPC) channels (7) and VK (TPK) channels (8). Cl and malate efflux through S-type and rapid (R-type) anion channels (4) and possibly CLC Cl/H+ antiporters (6) causes depolarization and drives K+ efflux through outward-rectifying K+ channels (5). At the vacuole membrane, SV (TPC1) channels (7) are Ca2+-activated and Ca2+-permeable voltage-dependent channels. VK (TPK) channels (8) are Ca2+ activated, are highly selective for K+, and are proposed to allow K+ release from the vacuole during stomatal closing. (Right) Mechanisms that function in guard cell ion uptake and stomatal opening. Blue light (10) activates phototropins (receptor/kinase), leading to stomatal opening. Signaling results in 14-3-3 binding to plasma membrane proton pumps (11), leading to hyperpolarization and acidification of the extracellular space. Hyperpolarization activates inward-rectifying K+ channels (12). At the vacuolar membrane, proton pumps (13) acidify the vacuole lumen and drive K+/H+ antiporters (14 ). Cl and malate may accumulate in the vacuole through anion channel and anion antiporters (15 ).