Abstract
Purpose
The purpose of this study was to assess the effect of fixation target parameters on fixation instability in strabismic monkeys.
Methods
One normal and three exotropic monkeys were presented with four differently shaped fixation targets, with three diameters, during monocular or binocular viewing. Fixation targets were white on a black background or vice versa. Binocular eye movements were recorded using the magnetic search coil technique and fixation stability quantified by calculating the bivariate contour ellipse area (BCEA).
Results
Fixation instability was greater in all the strabismic monkeys compared with the normal monkey. During monocular viewing, strabismic monkeys showed significantly greater instability in the covered eye compared to the fixating eye. Multifactorial ANOVA suggested statistically significant target parameter influences, although effect sizes were small. Thus, a disk-shaped target resulted in greater instability than other target shapes in the viewing eyes of the normal monkey and two of three strabismic monkeys. A similar target-shape effect was also observed in the covered eye. Least instability was elicited with a 0.5° target in the normal monkey and a 1.0° target in the strabismic monkeys, both in the viewing and the covered eye. Target/background polarity effects were idiosyncratic. In strabismic monkeys, stability of the fixating eye during binocular viewing was not different from the stability of the same eye during monocular viewing.
Conclusions
Abnormal drifts and nystagmus contribute to increased fixation instability in strabismic monkeys. Target parameters (shape and size) that influence fixation stability in a normal animal also affected fixation stability in our sample of strabismic monkeys.
Keywords: strabismus, nonhuman primate, fixation stability, BCEA
Small-amplitude involuntary eye movements produced during attempted visual fixation are called fixational eye movements. Microsaccades, drifts, tremors, and a recently described slow oscillatory eye movement are components of fixational eye movements.1,2 The primary role of fixational eye movements is perhaps to prevent visual fading that occurs as a neural adaptation to a stabilized image on the retina: the Troxler fading effect.3,4 Other studies have suggested that fixational eye movements are necessary to view fine detail and for the correct discrimination of even briefly presented low contrast stimuli.5,6 Conversely, excessively large eye movements during fixation (unstable fixation or fixation instability) can move the fovea away from the object of regard, leading to a decrement in visual performance. For example, unstable fixation interferes in tasks as varied as reading7,8 and shooting clay pigeons.9 Fixation stability in darkness is poorer than fixation stability measured when viewing a target in the light, suggesting that both visual and oculomotor processes are playing a role in maintaining stable fixation.10
Fixational instability is often a feature of visual disease. Thus, unstable fixation has been reported in ocular abnormalities such as strabismus, amblyopia,11–14 and macular diseases.15–19 Fixation instability in these cases includes oculomotor instabilities such as saccadic intrusions, manifest, and latent nystagmus.11,13
Certain parameters of the visual stimulus are known to influence fixation stability in normal humans and monkeys.20–23 For example, an increase in fixation target size can increase fixation instability in both humans20 and monkeys.21 Other studies16,23 have shown that the shape of the fixation target could affect fixation instability in normal human subjects. On the other hand, Steinman20 and McCamy et al.22 report that target luminance does not affect fixational eye movements and fixation instability. Ukwade and Bedell24 report a small effect of blur and no effect of contrast on fixation instability in normal subjects.
The overall goal of this study was to evaluate fixation instability of both eyes in strabismic monkeys and consider whether factors that appear to influence fixation instability in normals (e.g., target parameters), would also exert similar influence in strabismics. One possibility is that the influence of fixation target parameters on fixation instability in strabismus is significant and perhaps similar to that observed in normals. Alternatively, in the strabismic subject, the ongoing drifts and nystagmus eye movements could mask any potential influence of fixation target parameters. Our studies were performed in monkeys as they are excellent models for the human visual and oculomotor system, and strabismus in monkeys shows properties that are very similar to human strabismus.25–28 In the current study, our first aim was to assess whether manipulating fixation target shape, size, and background affects fixation instability of the viewing and deviated eye in strabismic monkeys and to compare these effects to the effects observed in a normal monkey. Our second aim was to compare fixation instability under monocular and binocular viewing conditions in the normal and strabismic monkeys.
Methods
Subjects and Rearing Paradigm
Fixation data were collected from three strabismic (SM1, SM2, and SM3) and one normal (NM) rhesus monkeys (Macaca Mulatta). Monkeys were made strabismic using one of two sensory methods: optical prism viewing (S1) or daily alternating monocular occlusion (AMO; SM2 and SM3).26,29–31 In the optical prism viewing procedure, infant monkeys viewed through a horizontal 20-diopter (D) prism placed in front of one eye and vertical 20-D prism placed in front of the other eye. These horizontal and vertical Fresnel prisms were fitted in a lightweight helmet-like device that the animal wore for the first 4 months of life starting from 1 to 2 days after birth. In the daily alternating monocular occlusion procedure, an occluding patch (opaque goggles or contact lens) is placed in front of one eye. The following day, the patch is switched to the other eye and thereafter the patch is alternated daily for a period of 4 months. Both these rearing paradigms disrupt the monkey's binocular vision during the critical period of visual and oculomotor development (binocular noncorrespondence in prism monkey and binocular deprivation in AMO). Disruption of binocular vision during this initial period leads to strabismus, as it is the critical period for development of eye alignment, stereopsis, and binocular sensitivity.32,33
Surgical Procedures
After special rearing, the animals were allowed to grow normally until they were approximately 4 years of age before starting behavioral experiments. Sterile surgical procedures performed under aseptic conditions using isoflurane anesthesia (1.25–2.5%) were used to stereotaxically implant a head stabilization post.34 In a second surgery, a scleral search coil was implanted in one eye using a procedure developed by Judge et al.35 Later in a third surgery, a second scleral search coil was implanted in the fellow eye. All procedures were performed in strict compliance with National Institutes of Health guidelines and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and the protocols were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Houston.
Construction and Presentation of Experimental Stimuli
Fixation targets that are commonly used for fixation research were constructed using MATLAB and the Cambridge Research Systems (CRS) toolbox.16,23 Visual stimuli were generated with the ViSaGe visual stimulus generator operated under computer control (Cambridge Research Systems, Cambridge, UK). Four target shapes (central cross, X; disk; optotype, %; and a combination of a disk and crosshair, disk+) (Fig. 1), each in three sizes (0.5°, 1.0°, and 2.0°) were presented with two polarities (white target on black background or black target on white background). Our choice of target shape was guided by the paper of Thaler et al.,23 who found that a disk target produced greatest instability and a disk+ target produced least instability. The X was included as it is a commonly used target shape. In our laboratory, we use 1°–2° % optotype during training and experiments in which the animals' had previously participated, therefore motivating our choice of including the % optotype for this study. White target luminance was 470 cd/m2 and black background luminance was 0.5 cd/m2. Both monocular and binocular viewing conditions were tested (right eye viewing, left eye viewing, and binocular viewing), yielding a total of 72 different stimulus combinations (4 shapes × 3 sizes × 2 polarities × 3 viewing conditions). Because eye movements were measured with search coils, it was possible to get eye position data from both eyes simultaneously even when one of the eyes was covered. During testing, each stimulus, selected in random order from the 72 possible conditions, was presented for 60 seconds in the center of a tangent screen at a distance of 114 cm from the monkey. After each fixation trial, monkeys were presented with a saccade stimulus (four to five saccade trials of 2–3 seconds each) to keep them alert, avoid adaptation to the fixation target position, and minimize effects of after images. Further, each dataset of 72 stimulus combinations was repeated five times (different days in different random order), yielding a total of 360 presentations for each animal.
Figure 1.

Shapes used as fixation targets: cross, disk, combination of disk and crosshair (disk+), % optotype.
Eye Movement Measurement, Data Acquisition, and Analysis
All animals were behaviorally trained in standard oculomotor tasks for several years prior to their participation in this study. Binocular eye position was measured using the magnetic scleral search coil technique. This method provides high spatial (<0.01°) resolution and therefore is well suited to study fixational eye movements.36,37 Eye coil signals were calibrated before each experimental session by rewarding the monkey with a small amount of juice for looking at a 1° optotype target projected at different positions (±15°) along the horizontal or vertical meridian. Calibration of each eye was performed independently during monocular viewing. To facilitate monocular viewing, the other eye was occluded with liquid crystal shutter goggles (Micron Technology, Boise, ID, USA) that were under computer control. At the time of the study, SM3 had a functional coil only in the left eye and so only data from this eye could be acquired and analyzed. This also reduced the number of different stimulus conditions to 48 in this monkey (because only two viewing conditions, monocular left eye viewing and binocular viewing, were testable).
Eye and target position data were processed with anti-aliasing filters (Krohn-Hite, Brockton, MA, USA) at 400 Hz prior to sampling at a frequency of 2.79 KHz with 12-bit precision (AlphaLabSnR, AlphaOmega Industries, Nazareth, Israel). Eye position data were further filtered with a finite impulse response software digital filter with a passband of 0–60 Hz prior to analysis of fixation. Epochs of fixation were selected by visual inspection of data, thereby excluding saccades, blinks, and any sections of data that the monkey was not looking at the target. Nystagmus (e.g., latent nystagmus), drifts, and microsaccades contribute to fixation instability and would not be removed by our selection method. An individual trial was accepted for further analysis only if the sum of the fixation epochs within the trial was greater than 10 seconds. Fixation data were then analyzed using custom MATLAB programs that calculated the bivariate contour ellipse area (BCEA). The BCEA is a metric that quantifies the area of the region over which eye positions fall during attempted fixation. Therefore, a smaller value for BCEA is indicative of greater fixation stability. The BCEA encompassing 68.2% of fixation points was calculated using the following equation38:
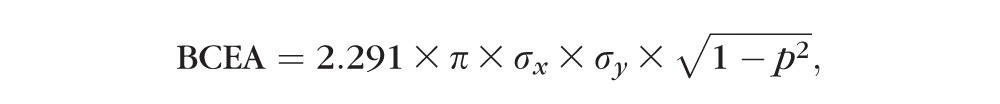 |
where σx = SD of horizontal eye position, σy = SD of vertical eye position, 2.291 is the χ2 value (2 df) corresponding to a probability of 0.68, and p is the Pearson product moment correlation coefficient of horizontal and vertical eye positions.
Statistical Analysis
Statistical analysis was performed using Minitab 16 statistical software. For each monkey, multifactorial (four-way) ANOVA was used to test the main effects of target shape, size, target/background polarity, and viewing conditions on BCEA of both the viewing and deviated eyes. Because the BCEAs are usually not normally distributed, we used a natural log transformation on these values to normalize the data prior to ANOVA. The multifactorial ANOVA design allowed us to also examine interaction effects between the variables. Only two-way interactions (i.e., interactions between any two pairs of target parameter variables yielding six additional comparisons) were examined because they were likely to be most meaningful and interpretable. All post hoc comparisons were performed using the Tukey test (95% confidence interval). For the post hoc analysis, to test the effects of one factor (shape for example), data across other factors (size, background, and viewing condition) were pooled. One-way ANOVA and paired t-tests were performed to compare BCEA values across monkeys and to compare viewing and nonviewing eye fixation instability.
Results
Properties of strabismus in SM1–SM3 are provided in Table 1. Briefly, the three monkeys were exotropic with strabismus angle varying from 10° to 25°. They all showed a dissociated vertical and horizontal deviation (DVD and DHD, respectively) and a small latent nystagmus (LN) similar to other monkeys reared under similar conditions.39–41 Also included in Table 1 are the means and ranges of fixation times that were analyzed in each 60-second trial and are provided to show that the animals provided sufficient data to analyze instability of fixation.
Table 1.
Strabismus Properties of Animals in the Study
Figure 2 shows a 15-second epoch of fixation in a normal monkey (left column traces) and in a strabismic monkey (SM1; right column traces). Both sets of data were collected as the animals monocularly viewed a white cross (X) target of size 1° against a black background. Note that the amplitude scale bars for the normal monkey data and the strabismic monkey data are different to enable visualization of fixational eye movements in both the normal and the strabismic. Stable fixation was observed in both eyes of the normal monkey with microsaccades on the order of approximately 0.5° and small amplitude drifts. The strabismic monkey showed significant instability due to increased drifts (predominantly in the horizontal plane) and nystagmus (predominantly in the vertical plane). Similar qualitative observations could be made in the other strabismic monkeys (data not shown).
Figure 2.
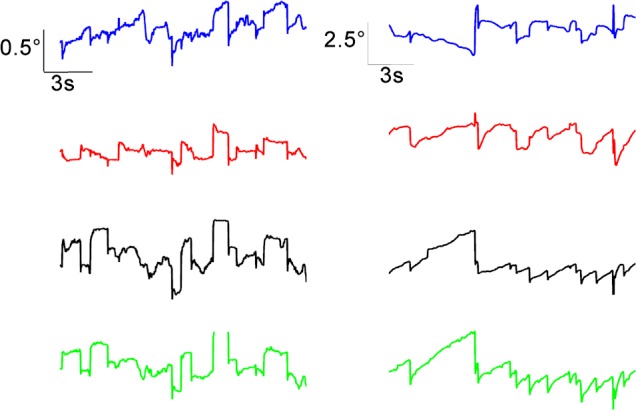
Fixational eye movements in the normal monkey (left column) and strabismic monkey SM1 (right column). Animals were monocularly fixating a 1° cross (X) for 15 seconds. Amplitude scales are different in two columns (normal: 0.5°; strabismic: 2.5°). Blue, left eye horizontal; red, right eye horizontal; black, left eye vertical; green, right eye vertical.
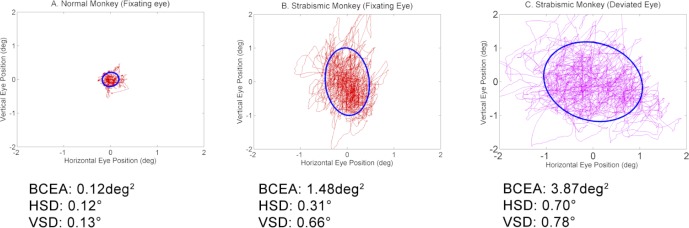
Figure 3 shows the two-dimensional dispersion of eye positions during fixation in the normal monkey and in a strabismic monkey (SM2). Also shown is the ellipse that contains 68.2% of the fixation points. The BCEA value is the area of the plotted ellipse. In these trials, the monkeys monocularly viewed a white disk target of diameter 1° against a black background (right eye viewing; left eye also deviated in the case of the strabismic monkey).
Figure 3.
Dispersion of eye movements during fixation of a 1° disk target in a normal monkey (A; right eye viewing) and in strabismic monkey SM2 (B, viewing right eye; C, nonviewing left eye). Also plotted are the ellipses that encompass 68.2% of fixation data points. Area of the plotted ellipses is the BCEA. For the normal monkey, the nonviewing eye BCEA and horizontal and vertical SD (not shown) were 0.20 deg2, 0.16°, and 0.19°.
The mean BCEAs across all monocular fixation conditions are plotted in Figure 4. A fundamental observation from Figures 3 and 4 is that BCEA values were significantly greater in the strabismic monkeys compared with the normal (1-way ANOVA on logBCEA and Tukey post hoc test; viewing eye: F[3,956] = 263.54, P < 0.001; nonviewing eye: F[2,717] = 867.31, P < 0.001) and that BCEA values in the fixating eye of the strabismic monkeys were significantly less than BCEA in the deviated eye (paired t-test on logBCEA; SM1: t[240] = −25.27, P < 0.001; SM2: t[240] = −12.93, P < 0.001). In the normal monkey also, the BCEAs in the fixating and covered eyes were statistically significantly different (paired t-test on logBCEA; NM: t[240] = −2.74, P = 0.007), but note that the actual mean difference was only 0.03 deg2.
Figure 4.
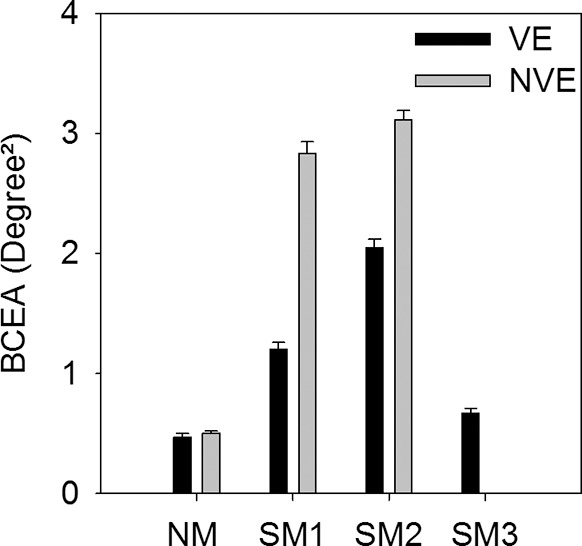
Mean BCEA across all left and right eye monocular viewing trials in normal and strabismic monkeys. VE, viewing eye; NVE, nonviewing eye.
Influence of Target Parameters on Fixation Stability
A principal goal of the study was to examine the influence of target parameters on gaze stability. Figure 5 shows the main effects of target shape, size, background, and viewing conditions on fixation instability of the viewing eye (Figs. 5A, 5C, 5E, 5G) and covered eye (Figs. 5B, 5D, 5F, 5H) in each monkey. As detailed in the methods, statistical analysis was performed on the logBCEA. Tables 2 and 3 summarize the results of the ANOVA and post hoc testing for the viewing eye (Table 2) and covered eye (Table 3). To preserve clarity of presentation of post hoc testing results, we only provide the largest (most unstable) and the smallest (least unstable) BCEA conditions instead of listing all significant differences. In general, target parameters showed significant but small magnitude effects on fixation instability.
Figure 5.
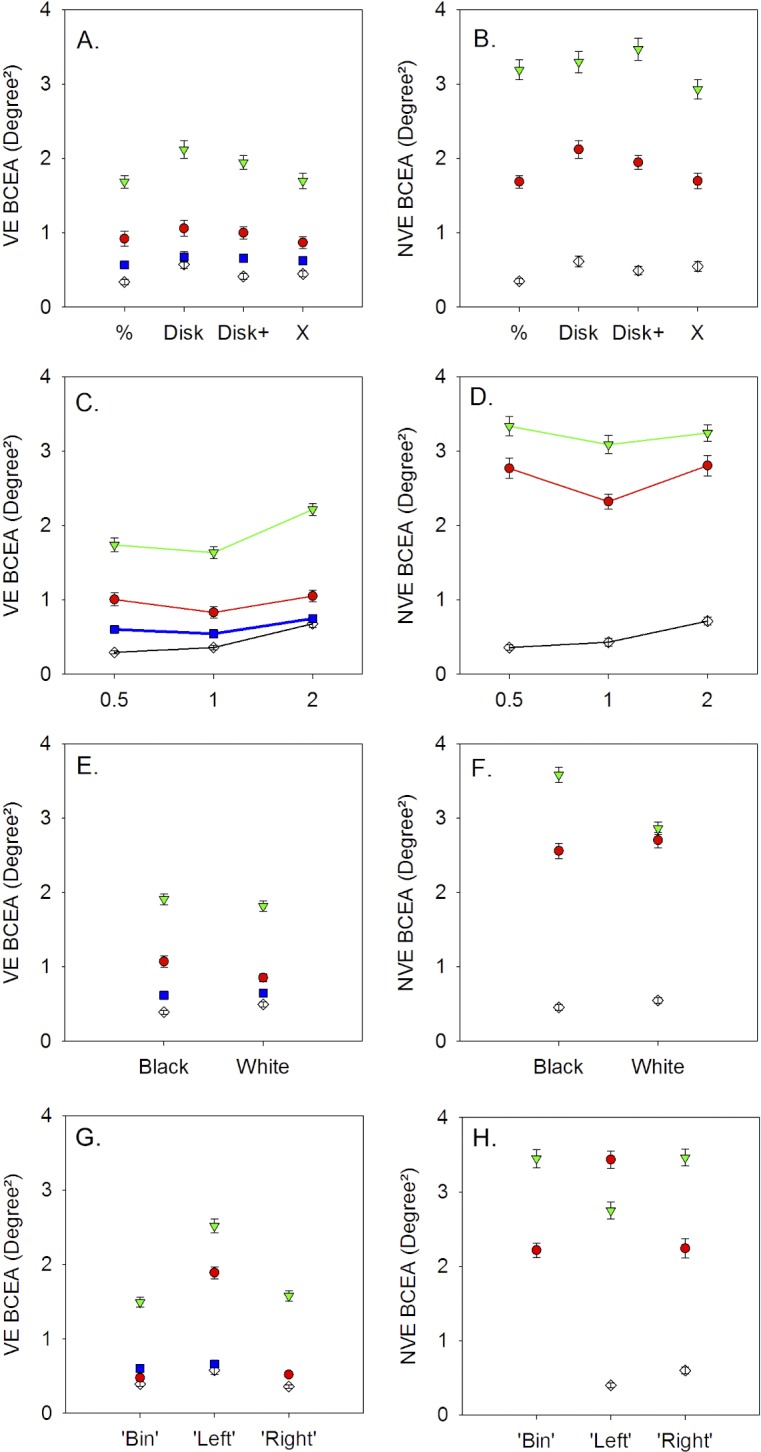
Influence of target shape (A, B), target size (C, D), background color (E, F), and viewing conditions (G, H) on BCEA of the viewing eye (A, C, E, G) and nonviewing eye (B, D, F, H) in normal and strabismic monkeys. NM, black unfilled diamonds; SM1, red circles; SM2, green triangles; SM3, blue squares; VE, viewing eye; NVE, nonviewing eye; ‘Bin’, binocular viewing; ‘Left’, left eye viewing; ‘Right’, right eye viewing. Symbols and error bars denote mean ± SE. In NM, only monocular viewing trials were used to calculate NVE BCEA. The NVE BCEA (B, D, F, H) and VE BCEA for the right eye (G) were not calculated for SM3 because this monkey did not have a functional coil in the right eye.
Table 2.
ANOVA Analysis of logBCEA Data From Viewing Eye
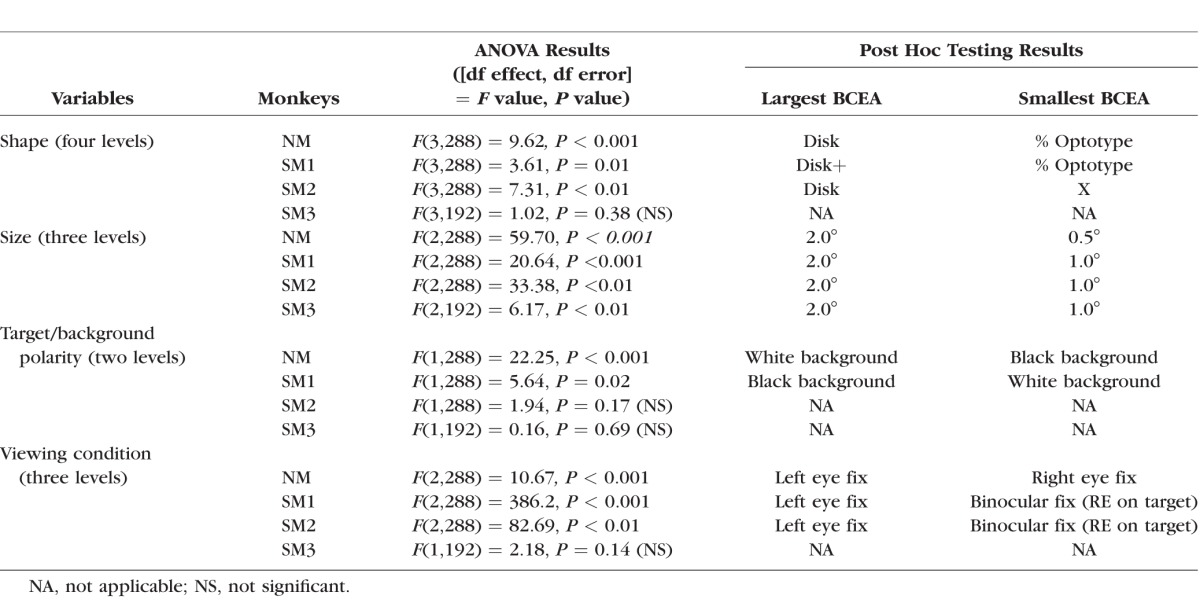
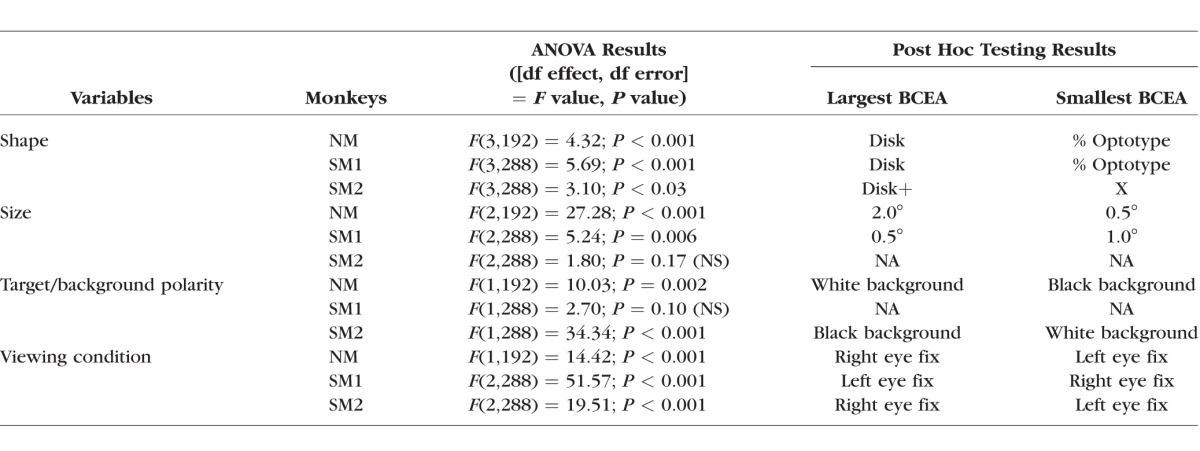
Table 3.
ANOVA Analysis of logBCEA Data From Nonviewing Eye
The ANOVA indicated a significant but small target shape effect in the viewing eye and covered eye BCEA (Figs. 5A, 5B; Tables 2, 3) in NM, SM1, and SM2 but not SM3 (also covered eye BCEA was not available in SM3). Post hoc testing revealed that the disk or disk+ target resulted in higher BCEA compared with other targets.
The main effect of target size on viewing eye BCEA was also significant with small effect size in all monkeys with greatest instability resulting from the 2° stimulus (Fig. 5C; Table 2). Post hoc testing indicated that the 0.5° target yielded smallest BCEA in NM but the 1° target yielded smallest BCEA in the strabismic monkeys. The target size effect on the nonviewing eye was significant in NM and SM1 (0.5° and 1.0° target resulted in smallest BCEA in NM and SM1, respectively) but not in SM2 (Fig. 5D; Table 3).
A significant but small background effect was observed in the viewing eye BCEA of NM and SM1 and in the nonviewing eye BCEA of NM and SM2 (Figs. 5E, 5F; Tables 2, 3). As shown in Tables 2 and 3, the background effects on the monkeys were rather idiosyncratic, as NM preferred a white target/black background and the strabismic monkeys (when significant) preferred a black target/white background.
Fixation Instability Under Monocular and Binocular Conditions
Under binocular viewing, the strabismic monkeys generally showed a preference for fixating the target with one eye over the other. This was easily distinguished from the eye and target feedback position signals because position data from one of the eyes were close to that of the target (denoted as the VE), whereas the position data from the other eye were deviated well away from the target (denoted as the NVE). For the normal monkey, it was not possible to determine which eye was used for fixation during binocular viewing, and therefore VE BCEA was calculated as the average of the right and left eye BCEAs. Also, NVE BCEA was not relevant during binocular viewing for the normal monkey.
Figures 5G and 5H show the main effects of monocular or binocular viewing on fixation stability. Note that the x-axis labels in these two panels denote the viewing condition and not the eye. For example, in Figure 5H (NVE BCEA), the data corresponding to ‘Left’ (x-axis label) are the BCEA of the nonviewing right eye during left eye viewing. The ANOVA analysis (Tables 2, 3) indicated that viewing condition was a significant factor in all monkeys. We were specifically interested in two aspects related to viewing condition: (1) do monkeys show significant differences in fixation instability under the two monocular viewing conditions; and (2) does binocular viewing influence fixation instability? The post hoc analysis allowed us to answer both questions. The first observation was that in all monkeys (not testable in SM3), one of the monocular viewing conditions always yielded greater instability compared with the other monocular viewing condition.
To address the second question, we examined the post hoc analysis output comparing the BCEA of the eye that the strabismic animal used for fixation during binocular viewing with the same eye BCEA during monocular viewing. For example, SM1 used his right eye for fixating the target during binocular viewing, and therefore BCEA of the right eye obtained during binocular viewing was compared with BCEA of the right eye obtained during monocular right eye viewing. Because there is no clear eye preference in the normal monkey, we took the average of the right eye and left eye BCEA during binocular viewing and compared it with the two monocular viewing conditions. The post hoc analysis revealed that for the strabismic monkeys, the binocular viewing VE BCEA was never significantly different from the corresponding monocular viewing VE BCEA (ANOVA Tukey post hoc testing, P > 0.05). The normal monkey showed a binocular viewing effect in that binocular viewing was better than left eye viewing but not right eye viewing. It should be noted that for the normal monkey, left eye viewing was less stable than right eye viewing. The above observations were also true for the nonviewing eye BCEA.
Interaction Effects Between Target Variables
Our four-way design allowed examination of statistical interaction effects between target variables. To simplify presentation, F values of only significant interactions are reported here. In NM, no significant interactions between the target parameters were identified for either the VE logBCEA or NVE logBCEA (P > 0.05). In the VE logBCEA of both SM1 and SM2, there were significant interactions between shape and size (SM1: F[6,288] = 5.30, P < 0.01; SM2: F[6,288] = 3.07, P < 0.01) and size and viewing condition (SM1: F[4,288] = 1.02, P < 0.01; SM2: F[4,288] = 5.90, P < 0.01). Visual examination of interaction plots between shape and size suggested that the disk and disk+ shape showed relatively increased instability for the 2° target size compared with the other shapes, suggesting that the shape effect identified earlier may be specific to the larger target size. SM3 did not show a statistically significant interaction between shape and size, although the trend (based on visual examination of interaction plots) was similar as in the other strabismic monkeys. Similar visual examination of interaction plots between size and viewing condition suggested that there was relatively less influence of target size when the animal viewed with his worse eye (i.e., left eye for both SM1 and SM2) than if they viewed with their better eye or if they viewed binocularly. Interaction analysis of the NVE logBCEA showed shape and size interactions in SM1 (F[6,288] = 6.46, P < 0.01) and size and viewing condition interactions in SM2 (F[4,288] = 3.83, P < 0.01). Once again, note that the main effect sizes are small to begin with, and therefore the functional significance of the interaction effects are not clear.
Discussion
In this study, we evaluated fixation instability in strabismic monkeys with the additional goal of examining whether target parameters and viewing conditions can influence the BCEA metric. Our main finding is that fixation in strabismic monkeys is more unstable than in the normal. Although effect sizes were small, stimulus factors that influence fixation instability in the normal animal are also effective in influencing fixation instability in strabismic monkeys. Here we discuss our results with the goal of further understanding monocular and binocular influences on fixation instability in normals and strabismics.
Methodologic Considerations
There are many choices for analyzing dispersion of eye movements during fixation, of which the BCEA is the most popular.42 However, one caveat is that the BCEA is predicated on the normal distribution of horizontal and vertical components of eye positions and fixational eye movements often deviate from normality, especially when the data matrices are large due to high sampling rates.38,42 We used the BCEA as our metric of choice because in our opinion it is the most intuitive metric to examine fixation stability. Further, this metric has more or less become the standard in the field allowing our data to be compared with a host of other human and monkey studies published in the literature. Analysis of microsaccades is also found in studies of fixation stability. However, we did not separately analyze microsaccades because of the presence of nystagmus in all our strabismic monkeys. For example, it is not yet known if the sensory and motor mechanisms that drive microsaccades are also the same ones that drive nystagmus quick phases. On the other hand, using the BCEA to analyze fixation stability provides an overall metric that includes the contributions of all fixational eye movement components.
One question is whether the scleral search coils, usually considered the gold standard for measuring eye movements, could affect fixation stability due to the very small weight of the coils. We do not believe the coils were a factor because of two reasons. First, we might expect any disruption due to coils to be similar across the different stimulus conditions within any monkey and across normal and strabismic monkeys because the coil design and placement in all the monkeys is the same. The statistical comparisons we made were within a monkey for the different stimuli, and in these comparisons, any effect of the coils would be constant. Second, there is no indication that the normal monkey shows significant fixation instability, which might be expected if the coils were a problem.
We used two different sensory rearing conditions to induce strabismus in our monkeys. However, due to the small sample size of strabismic monkeys, we did not attempt to examine whether the rearing condition has specific influence on fixation stability. We suggest that grouping the differently reared animals together is valid because they share the common features of human strabismus and neither sensory rearing paradigm predisposes the animal toward fixation instability (unlike, for example, a surgical strabismus model that could alter proprioceptive receptors and therefore influence fixation stability). Despite the relatively small sample size in our study (unfortunate reality in most monkey studies due to practical considerations), establishing these effects in strabismic monkeys help to validate the monkey model for the human condition and can provide a framework for future neurophysiological investigation.
Fixation Stability in Strabismic Monkeys in Comparison to the Normal Monkey
Although we had only one normal monkey in our study, results from this animal agree with published data in the literature. For example, when this animal monocularly fixated a 0.5° target, we found the mean right eye BCEA value was 0.24 deg2, and the corresponding mean SDs of horizontal and vertical positions were 0.17° and 0.19°, respectively. These values are similar to measurements estimated from a publication by Motter et al., where they examined fixation stability in monkeys.21 In another study, monkeys fixated a 2.5-arc minutes target and showed BCEA values ranging from 150 to 1214 minutes of arc2, with horizontal standard deviation (HSD) ranging from 4.2 to 7.5 arc minutes and vertical standard deviation (VSD) ranging from 4.2 to 22.6 arc minutes.43
Overall fixation in strabismic monkeys was more unstable than in the normal monkey, and fixation instability was greater in the deviated eye than in the fixating eye. These general findings are in accordance with the reports of Gonzalez et al. and Subramanian et al. on fixation instability in humans with strabismus and amblyopia.12,14 Ciuffreda et al.11,44 reported abnormal fixational eye movements in strabismus and amblyopia that included increased drift, saccadic intrusions, and latent nystagmus. We also observed all these abnormal eye movements in our strabismic monkeys, most likely accounting for the larger BCEAs values.
Fixation in strabismic monkeys could also be rendered unstable by factors such as reduced acuity and contrast sensitivity, although in the present study only bright, high-contrast targets were used. We did not measure visual acuity in our monkeys, but the animals included in the study were all part of other oculomotor studies in our laboratory and were able to perform saccadic and smooth-pursuit oculomotor tasks with either eye viewing, indicating that they did not have severe amblyopia. However, visual acuity and stereoacuity is correlated with BCEA in subjects with strabismus and anisometropic amblyopia,12,45 suggesting that our strabismic monkeys' visual acuity could be less than that of the normal animal. On the other hand, another study of amblyopic subjects did not find a clear relationship between visual acuity and BCEA,14 suggesting that the relationship between BCEA and visual acuity is not yet resolved.
Influence of Monocular or Binocular Viewing on Fixation Stability
A consistent finding in all the monkeys was that one of the monocular viewing conditions resulted in larger fixation instability in both the viewing and covered eyes than the other. Although statistically significant, the magnitude of the difference was very small in the normal monkey. In strabismic monkeys, the differences were large (Figs. 5G, 5H), and we took this observation to mean that one of the eyes was relatively amblyopic compared with the other eye, because other studies that actually measured both visual acuity and fixation stability in amblyopes have shown that BCEA values are larger when subjects viewed with their amblyopic eye.12,14,45 The second observation was that during binocular viewing, the strabismic monkeys preferred viewing with the eye that yielded the better stability. Moreover, stability of the fixating eye during binocular viewing was not significantly better than the stability of the same eye during the equivalent monocular viewing condition. This finding is similar to a study by Cuiffreda et al.11 and in some studies in patients with retinal disease46 but perhaps slightly different from the observation of Gonzalez et al.,14 who found some binocular summation when the nonamblyopic eye viewed the target (i.e., binocular viewing was better than monocular nonamblyopic eye viewing). In trying to understand the source of the difference, we suggest that it may be because our rather small sample of monkeys were all strabismic, whereas the patients in the study of Gonzalez et al. were a mix of strabismic and anisometropic amblyopes. Additional studies are necessary to understand if binocular summation leading to better fixation stability during nonamblyopic eye viewing is different in anisometropic versus strabismic amblyopes.
Target Parameter Influences on Fixation Instability in Normal and Strabismic Monkeys
Previous studies on normal humans suggest that visual parameters such as contrast, luminance, blur, and color exert little or no influence on fixation instability.20,22,24 However, other studies have shown that target shape does indeed matter.16,23 Thus, Thaler et al.23 report, in normal subjects, that a disk target resulted in higher dispersion of eye movements and increased microsaccade rate compared with other shapes that were tested. This shape was replicated in our study, and indeed, the normal monkey showed higher BCEA values with the disk shape compared with other shapes. Bellman et al.16 report that fixation instability in normal subjects is significantly greater for peri-central fixation targets (large four-point diamond) compared with targets that provide central stimulation (1° cross). We also observed the greatest BCEA values with fixation targets that provided least central stimulation such as the disk target. We included the % target to assess whether extensive training might influence fixation instability. Although this target did yield the smallest BCEA in two of the monkeys (NM and SM1), the effect sizes are too small to be confident of a training effect. Target size influences were also observed in the normal monkey data in our study. Thus, a target size of 0.5° yielded greatest stability, and a target size of 2° was least stable. The target size effect is in basic agreement with previous studies in normal humans and monkeys, all of which showed increased SDs of horizontal or vertical eye positions for larger targets.10,20,21,23,47,48 A study by McCamy et al.22 found that microsaccade rate decreased linearly and microsaccade magnitude increased linearly with target size in normal human subjects, and this could be the reason for increased BCEA values in normal subjects or monkeys.
We were surprised to find that the target shape and size influences were also significant in the strabismic monkeys and that these effects were similar to the effects in the normal. For example, the strabismic animals also showed the largest VE BCEA values for the disk target compared with the other targets (statistically significant in two of three animals). In strabismic monkeys, the smallest VE BCEA was observed with the 1.0° target, and the BCEA increased for 0.5° and 2.0° targets. From our results, it appears that abnormal drifts and nystagmus eye movements and possible reduced visual acuity (amblyopia) do not mask the influence of fixation target parameters. Note, however, that, in the strabismic monkeys, the improvement in BCEA due to the best target shape or best target size is quite small, and fixation stability is still significantly worse than in the normal monkey. In fact, the effect sizes due to target parameters were small in the normal monkey as well. Therefore, the functional significance of the target-mediated effects is unclear in both the normal and strabismic subjects.
Mechanisms Underlying Fixation Instability
Not a lot is known about the mechanisms underlying instability of fixation. In fact, the utility of fixational eye movements, specifically microsaccades, was once questioned.49,50 However, more recent work has indeed shown rather conclusively that both microsaccades and drift components of fixational eye movements are critical for vision.3,6,51 A recently published mathematical model for fixational eye movements suggests that the occurrence of both drift and microsaccades can be explained as a self-avoiding random walk (a random sampling of space while avoiding areas previously sampled) in a region of space including and surrounding the fixation target whose image presumably lies on the fovea.52 An attractive feature of this model is that it fits in well with a recently proposed neurophysiological mechanism for microsaccade generation that suggests that an imbalance in activity between the right and left rostral superior colliculi can result in the generation of contraversive microsaccades.53,54 It is not clear what might cause the imbalance, but possibilities include that the imbalance is the consequence of the drift component of fixation, a “noisy” oculomotor system, or “noisy” visual inputs to the oculomotor system.
How can we extrapolate some of these concepts to understand increased fixation instability in strabismus/amblyopia and to the target mediated changes that we observed in our study? One framework would be to consider that early visual deprivation such as in strabismus and amblyopia renders the neural visual system to be more noisy.55,56 Additional support for this concept comes from some recent work that shows that receptive subfield maps in V2 are disorganized in amblyopic monkeys.57 The implication is that such disorganization in early visual structures will lead to increasingly poor discrimination of signals in downstream structures (e.g., in V4 due to poor signal-to-noise ratio of V2 inputs) and thereby eventually lead to visual perceptual deficits including positional uncertainty.58,59 These deficits in visual brain areas could in turn lead to oculomotor deficits such as fixation instability, for example, due to noisy input to the rostral superior colliculus.
Based on our finding that target shape and size influences fixation instability in both normal and strabismic monkeys, one could speculate that the changes in BCEA due to target shape and size are driven by areas of the brain that are relatively unaffected by early visual deprivation and the subsequent strabismus and amblyopia. Animal studies have shown that several fundamental properties of V1 (but not V2 or other downstream neurons) such as orientation bias, spatial resolution, and optimal spatial frequency are relatively unaffected or only mildly affected in amblyopia.60,61
Acknowledgments
The authors thank Harold Bedell and Julia Benoit for helpful discussion on statistical methodology, Darren Koenig for help with using Minitab software, and Ernest Baskin for technical assistance with the animals.
Supported by National Institutes of Health Grants R01-EY015312 and R01-EY022723 and UHCO Core Grant P30 EY 07551.
The authors alone are responsible for the content and writing of the paper.
Disclosure: O.H. Pirdankar, None; V.E. Das, None
References
- 1. Martinez-Conde S,, Macknik SL,, Troncoso XG,, Hubel DH. Microsaccades: a neurophysiological analysis. Trends Neurosci. 2009; 32: 463–475. [DOI] [PubMed] [Google Scholar]
- 2. Pansell T,, Zhang B,, Bolzani R,, Ygge J. Slow oscillatory eye movement during visual fixation. Exp Brain Res. 2011; 209: 1–8. [DOI] [PubMed] [Google Scholar]
- 3. Martinez-Conde S,, Macknik SL,, Troncoso XG,, Dyar TA. Microsaccades counteract visual fading during fixation. Neuron. 2006; 49: 297–305. [DOI] [PubMed] [Google Scholar]
- 4. Costela FM,, McCamy MB,, Macknik SL,, Otero-Millan J,, Martinez-Conde S. Microsaccades restore the visibility of minute foveal targets. Peer J. 2013; 1:e119. [DOI] [PMC free article] [PubMed]
- 5. Rucci M,, Desbordes G. Contributions of fixational eye movements to the discrimination of briefly presented stimuli. J Vis. 2003; 3: 852–864. [DOI] [PubMed] [Google Scholar]
- 6. Ko HK,, Poletti M,, Rucci M. Microsaccades precisely relocate gaze in a high visual acuity task. Nat Neurosci. 2010; 13: 1549–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Amore FM,, Fasciani R,, Silvestri V,, et al. Relationship between fixation stability measured with MP-1 and reading performance. Ophthalmic Physiol Opt. 2013; 33: 611–617. [DOI] [PubMed] [Google Scholar]
- 8. Crossland MD,, Culham LE,, Rubin GS. Fixation stability and reading speed in patients with newly developed macular disease. Ophthalmic Physiol Opt. 2004; 24: 327–333. [DOI] [PubMed] [Google Scholar]
- 9. Di Russo F,, Pitzalis S,, Spinelli D. Fixation stability and saccadic latency in elite shooters. Vision Res. 2003; 43: 1837–1845. [DOI] [PubMed] [Google Scholar]
- 10. Sansbury RV,, Skavenski AA,, Haddad GM,, Steinman RM. Normal fixation of eccentric targets. J Optical Soc Am. 1973; 63: 612–614. [DOI] [PubMed] [Google Scholar]
- 11. Ciuffreda KJ,, Kenyon RV,, Stark L. Fixational eye movements in amblyopia and strabismus. J Am Optom Assoc. 1979; 50: 1251–1258. [PubMed] [Google Scholar]
- 12. Subramanian V,, Jost RM,, Birch EE. A quantitative study of fixation stability in amblyopia. Invest Ophthalmol Vis Sci. 2013; 54: 1998–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schor C,, Hallmark W. Slow control of eye position in strabismic amblyopia. Invest Ophthalmol Vis Sci. 1978; 17: 577–581. [PubMed] [Google Scholar]
- 14. Gonzalez EG,, Wong AM,, Niechwiej-Szwedo E,, Tarita-Nistor L,, Steinbach MJ. Eye position stability in amblyopia and in normal binocular vision. Invest Ophthalmol Vis Sci. 2012; 53: 5386–5394. [DOI] [PubMed] [Google Scholar]
- 15. Crossland MD,, Rubin GS. The use of an infrared eyetracker to measure fixation stability. Optom Vis Sci. 2002; 79: 735–739. [DOI] [PubMed] [Google Scholar]
- 16. Bellmann C,, Feely M,, Crossland MD,, Kabanarou SA,, Rubin GS. Fixation stability using central and pericentral fixation targets in patients with age-related macular degeneration. Ophthalmology. 2004; 111: 2265–2270. [DOI] [PubMed] [Google Scholar]
- 17. Dunbar HM,, Crossland MD,, Rubin GS. Fixation stability: a comparison between the Nidek MP-1 and the Rodenstock scanning laser ophthalmoscope in persons with and without diabetic maculopathy. Invest Ophthalmol Vis Sci. 2010; 51: 4346–4350. –43. [DOI] [PubMed] [Google Scholar]
- 18. Kumar G,, Chung ST. Characteristics of fixational eye movements in people with macular disease. Invest Ophthalmol Vis Sci. 2014; 55: 5125–5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gonzalez EG,, Tarita-Nistor L,, Mandelcorn ED,, Mandelcorn M,, Steinbach MJ. Fixation control before and after treatment for neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011; 52: 4208–4213. [DOI] [PubMed] [Google Scholar]
- 20. Steinman RM. Effect of target size luminance and color on monocular fixation. J Optical Soc Am. 1965; 55:1158.
- 21. Motter BC,, Poggio GF. Binocular fixation in the rhesus monkey: spatial and temporal characteristics. Exp Brain Res. 1984; 54: 304–314. [DOI] [PubMed] [Google Scholar]
- 22. McCamy MB,, Najafian Jazi A,, Otero-Millan J,, Macknik SL,, Martinez-Conde S. The effects of fixation target size and luminance on microsaccades and square-wave jerks. PeerJ. 2013; 1:e9. [DOI] [PMC free article] [PubMed]
- 23. Thaler L,, Schutz AC,, Goodale MA,, Gegenfurtner KR. What is the best fixation target? The effect of target shape on stability of fixational eye movements. Vision Res. 2013; 76: 31–42. [DOI] [PubMed] [Google Scholar]
- 24. Ukwade MT,, Bedell HE. Stability of oculomotor fixation as a function of target contrast and blur. Optom Vis Sci. 1993; 70: 123–126. [DOI] [PubMed] [Google Scholar]
- 25. Tychsen L,, Leibole M,, Drake D. Comparison of latent nystagmus and nasotemporal asymmetries of optokinetic nystagmus in adult humans and macaque monkeys who have infantile strabismus. Strabismus. 1996; 4: 171–177. [DOI] [PubMed] [Google Scholar]
- 26. Das VE. Alternating fixation and saccade behavior in nonhuman primates with alternating occlusion-induced exotropia. Invest Ophthalmol Vis Sci. 2009; 50: 3703–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Joshi AC,, Das VE. Responses of medial rectus motoneurons in monkeys with strabismus. Invest Ophthalmol Vis Sci. 2011; 52: 6697–6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Das VE. Responses of cells in the midbrain near-response area in monkeys with strabismus. Invest Ophthalmol Vis Sci. 2012; 53: 3858–3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Watanabe I,, Bi H,, Zhang B,, et al. Directional bias of neurons in V1 and V2 of strabismic monkeys: temporal-to-nasal asymmetry? Invest Ophthalmol Vis Sci. 2005; 46: 3899–3905. [DOI] [PubMed] [Google Scholar]
- 30. Crawford ML,, von Noorden GK. Optically induced concomitant strabismus in monkeys. Invest Ophthalmol Vis Sci. 1980; 19: 1105–1109. [PubMed] [Google Scholar]
- 31. Tusa RJ,, Mustari MJ,, Das VE,, Boothe RG. Animal models for visual deprivation-induced strabismus and nystagmus. Ann N Y Acad Sci. 2002; 956: 346–360. [DOI] [PubMed] [Google Scholar]
- 32. Boothe RG,, Dobson V,, Teller DY. Postnatal development of vision in human and nonhuman primates. Annu Rev Neurosci. 1985; 8: 495–545. [DOI] [PubMed] [Google Scholar]
- 33. Kiorpes L. Visual development in primates: neural mechanisms and critical periods. Dev Neurobiol. 2015; 75: 1080–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Adams DL,, Economides JR,, Jocson CM,, Horton JC. A biocompatible titanium headpost for stabilizing behaving monkeys. J Neurophysiol. 2007; 98: 993–1001. [DOI] [PubMed] [Google Scholar]
- 35. Judge SJ,, Richmond BJ,, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980; 20: 535–538. [DOI] [PubMed] [Google Scholar]
- 36. Collewijn H,, van der Mark F,, Jansen TC. Precise recording of human eye movements. Vision Res. 1975; 15: 447–450. [DOI] [PubMed] [Google Scholar]
- 37. Robinson DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng. 1963; 10: 137–145. [DOI] [PubMed] [Google Scholar]
- 38. Timberlake GT,, Sharma MK,, Grose SA,, Gobert DV,, Gauch JM,, Maino JH. Retinal location of the preferred retinal locus relative to the fovea in scanning laser ophthalmoscope images. Optom Vis Sci. 2005; 82: 177–185. [DOI] [PubMed] [Google Scholar]
- 39. Agaoglu MN,, LeSage SK,, Joshi AC,, Das VE. Spatial patterns of fixation in monkeys with strabismus. Invest Ophthalmol Vis Sci. 2014; 55: 1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Das VE,, Fu LN,, Mustari MJ,, Tusa RJ. Incomitance in monkeys with strabismus. Strabismus. 2005; 13: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Joshi AC,, Das VE. Muscimol inactivation of caudal fastigial nucleus and posterior interposed nucleus in monkeys with strabismus. J Neurophysiol. 2013; 110: 1882–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Castet E,, Crossland M. Quantifying eye stability during a fixation task: a review of definitions and methods. Seeing Perceiving. 2012; 25: 449–469. [DOI] [PubMed] [Google Scholar]
- 43. Skavenski AA,, Robinson DA,, Steinman RM,, Timberlake GT. Miniature eye movements of fixation in rhesus monkey. Vision Res. 1975; 15: 1269–1273. [DOI] [PubMed] [Google Scholar]
- 44. Ciuffreda KJ,, Kenyon RV,, Stark L. Increased drift in amblyopic eyes. Br J Ophthalmol. 1980; 64: 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chung ST,, Kumar G,, Li RW,, Levi DM. Characteristics of fixational eye movements in amblyopia: limitations on fixation stability and acuity? Vision Res. 2015; 114: 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tarita-Nistor L,, Brent MH,, Steinbach MJ,, Gonzalez EG. Fixation stability during binocular viewing in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011; 52: 1887–1893. [DOI] [PubMed] [Google Scholar]
- 47. Murphy J, Brian GMH,, Steinman R.. Simple forms and fluctuations of the line of sight: implications for motor theories of form processing. Percept Psychophys. 1974; 16: 557–563. [Google Scholar]
- 48. Zhang B,, Pansell T,, Ygge J,, Bolzani R. Visual influence on the slow oscillatory eye movement discovered during a visual fixation task. Vision Res. 2011; 51: 2139–2144. [DOI] [PubMed] [Google Scholar]
- 49. Kowler E,, Steinman RM. Small saccades serve no useful purpose: reply to a letter by R. W. Ditchburn. Vision Res. 1980; 20: 273–276. [DOI] [PubMed] [Google Scholar]
- 50. Kowler E,, Steinman RM. Miniature saccades: eye movements that do not count. Vision Res. 1979; 19: 105–108. [DOI] [PubMed] [Google Scholar]
- 51. Cherici C,, Kuang X,, Poletti M,, Rucci M. Precision of sustained fixation in trained and untrained observers. J Vis. 2012; 12( 6): 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Engbert R,, Mergenthaler K,, Sinn P,, Pikovsky A. An integrated model of fixational eye movements and microsaccades. Proc Natl Acad Sci U S A. 2011; 108: E765–E770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Goffart L,, Hafed ZM,, Krauzlis J. Visual fixation as equilibrium: evidence from superior colliculus inactivation. J Neurosci. 2012; 32: 10627–10636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hafed ZM,, Goffart L,, Krauzlis RJ. A neural mechanism for microsaccade generation in the primate superior colliculus. Science. 2009; 323: 940–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Levi DM. Linking assumptions in amblyopia. Vis Neurosci. 2013; 30: 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Levi DM,, Klein SA,, Chen I. The response of the amblyopic visual system to noise. Vision Res. 2007; 47: 2531–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tao X,, Zhang B,, Shen G,, et al. Early monocular defocus disrupts the normal development of receptive-field structure in V2 neurons of macaque monkeys. J Neurosci. 2014; 34: 13840–13854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Levi DM,, Klein SA,, Yap YL. Positional uncertainty in peripheral and amblyopic vision. Vis Res. 1987; 27: 581–597. [DOI] [PubMed] [Google Scholar]
- 59. Hess RF,, Holliday IE. The spatial localization deficit in amblyopia. Vis Res. 1992; 32: 1319–1339. [DOI] [PubMed] [Google Scholar]
- 60. Bi H,, Zhang B,, Tao X,, Harwerth RS,, Smith EL,, III, Chino YM. Neuronal responses in visual area V2 (V2) of macaque monkeys with strabismic amblyopia. Cereb Cortex. 2011; 21: 2033–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shooner C,, Hallum LE,, Kumbhani RD,, et al. Population representation of visual information in areas V1 and V2 of amblyopic macaques. Vis Res. 2015; 114: 56–167. [DOI] [PMC free article] [PubMed] [Google Scholar]