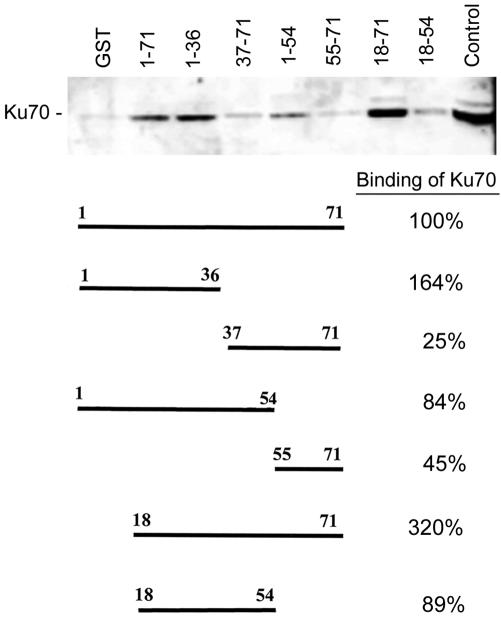
FIG. 7.
Analysis of agnoprotein-Ku70 interaction by using GST-agnoprotein fusion proteins. To see if Ku70 and the JCV agnoprotein could directly interact, we performed binding assays with protein extracts from U-87MG human glioblastoma cells that were negative for agnoprotein. Protein complexes were isolated by using glutathione-Sepharose 4B beads as described in Materials and Methods and were resolved by SDS-PAGE, followed by Western blotting with an anti-Ku70 antibody. The top panel shows a Western blot of the Ku70 that was pulled down by each of the GST-agnoprotein deletion mutant fusion proteins. The structure of each deletion mutant and its relative percentage of Ku70 binding are shown in the bottom panel. The lane on the far left is a negative control with GST only; the lane on the far right is a positive control with an unfractionated U-87MG cell extract. The relative intensities of the bands on the autoradiograph were measured by densitometry and the relative binding of Ku70 to each of the agnoprotein mutants was calculated as follows. The intensity of the GST-alone band (negative control) was subtracted from the intensity of each of the experimental lanes. The ratio of each of the bands relative to the full-length positive-control lane (1-71) was calculated and expressed as a percentage.