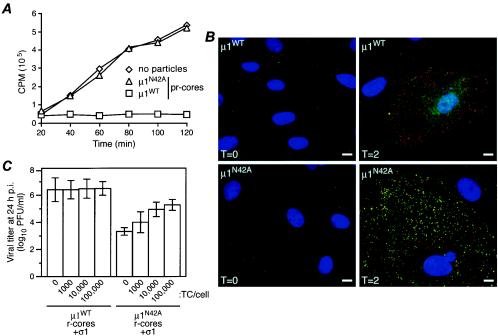
FIG. 6.
Behaviors of μ1N42A recoated particles in cells. (A) Coentry of α-sarcin during viral infection. L929 cells were incubated with μ1WT or μ1N42A pr-cores plus σ1 at 4°C to allow attachment, washed with methionine-free medium containing [35S]methionine-cysteine with or without α-sarcin (50 μg/ml), and incubated at 37°C for different times, as indicated. The cells were then lysed, and macromolecules were concentrated by trichloroacetic acid precipitation. The radioactivity (in counts per minute) in the washed precipitates was measured by scintillation counting. The values shown represent the average of two experiments, each done in duplicate. (B) Antibody-detected structural changes in infected cells. CV1 cells were infected with μ1WT pr-cores plus σ1 (top row) or μ1N42A pr-cores plus σ1 (bottom row) (50,000 particles per cell) and then fixed at either 0 (left column) or 2 (right column) h p.i. The fixed cells were coimmunostained with a core-specific rabbit polyclonal antiserum followed by Alexa 594-conjugated goat anti-rabbit immunoglobulin G (red) and the μ1-specific mouse MAb 4A3 followed by Alexa 488-conjugated goat anti-mouse immunoglobulin G (green). Cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (blue). Samples were examined by fluorescence microscopy. (C) Rescue of infectivity by top component. L929 cell monolayers were coinfected with μ1WT or μ1N42A r-cores plus σ1 (1,000 per cell) plus top component particles (0, 1,000, 10,000, or 100,000 per cell). After 24 h at 37°C, the cells were lysed, and infectious titers were determined by plaque assay. Samples of cells infected with each amount of top component particles alone were identically generated and analyzed in parallel. Within each experiment, the latter titers were subtracted from the respective former titers to correct for any residual infectivity of the top component. Each bar represents the mean ± standard deviation of the results from three separate experiments.