Abstract
Exendin-4 (ex-4) is a long-acting glucagon-like peptide-1 receptor (GLP-1R) agonist which exerts beneficial effects on glycemic control and promotes cell viability. In the present study, we investigated the anti-apoptotic effects of ex-4, as well as the potential mechanisms responsible for these effects in rat bone marrow-derived mesenchymal stem cells (BM-MSCs) under conditions of oxygen, glucose and serum deprivation (OGD). The apoptosis of the MSCs was induced by subjecting the cells to OGD conditions for 4 h and was detected by Annexin V/PI and Hoechst 33258 staining. The MSCs were pre-conditioned with ex-4 for 12 h prior to being subjected to OGD conditions, and the expression levels of an apoptotic marker (cleaved caspase-3), endoplasmic reticulum (ER) stress markers [phosphorylated (p-)protein kinase RNA-like endoplasmic reticulum kinase (PERK), PERK, binding immunoglobulin protein (BIP), activating transcription factor 4 (ATF-4) and C/EBP homologous protein (CHOP)], as well as those of a survival marker (Bcl-2) were measured by western blot analysis. Furthermore, the mRNA levels of ATF-4 and CHOP were determined by RT-qPCR. ELISA was used to examine the activity of intracellular cAMP. Moreover, the GLP-1R antagonist, exendin9-39 (ex9-39), the protein kinase A (PKA) inhibitor, H89, and small interfering RNA (siRNA) targeting rat ATF-4 and CHOP were co-incubated with the MSCs. The apoptotic rate was markedly diminished following pre-conditioning with ex-4 in a dose-dependent manner (P<0.05). The ER stress markers, p-PERK, BIP, ATF-4 and CHOP, were upregulated in the cells subjected to OGD conditions. Ex-4 pre-conditioning significantly decreased the mRNA and protein levels of ATF-4 and CHOP (P<0.05), and increased the activity of intracellular cAMP (P<0.05). Furthermore, the anti-apoptotic effects of ex-4 were almost reversed by treatment with either H89 or ex9-39 (P<0.05); transfection with siRNA-CHOP significantly reduced the apoptotic rate of the MSCs and did not impair the cytoprotective effects of ex-4. Taken together, these findings suggest that ex-4 protects rat BM-MSCs from OGD-induced apoptosis through the activation of the PKA/cAMP pathway and the attenuation of the ER stress signaling pathway. Ex-4 may thus prove to be a therapeutic agent with the potential to improve the viability of MSCs in the ischemic milieu, and consequently, to optimize the therapeutic effects of MSC therapy in acute myocardial infarction.
Keywords: mesenchymal stem cells, apoptosis, endoplasmic reticulum stress, exendin-4, ischemia
Introduction
Acute myocardial infarction (AMI) is caused by the sudden blockage of the coronary blood supply and leads to the hibernation and irreversible death of cardiomyocytes. It is usually followed by myocardial fibrosis, which results in decreased ventricular compliance, ventricular dilatation, and eventually, heart failure (1). Cell therapy using mesenchymal stem cells (MSCs) to improve the viability of hibernated cardiomyocytes and reduce fibrosis is an attractive prospect. Several clinical trials have highlighted the therapeutic effects of MSC transplantation, by reducing the size of the infarct area and improving the left ventricular ejection fraction (2,3). However, as previously demonstrated in a rat model of AMI, MSC-based therapy only achieved short-term benefits rather than a long-term impact on cardiac function (4). This may be explained by the findings of an in vivo animal study which revealed that less than 1% of engrafted MSCs had survived by day 4 following transplantation (5). The ischemic microenvironment, together with risk factors, including anoxia, as well as serum and glucose deficiency, contribute to the death of transplanted MSCs, by activating cellular signaling mechanisms, such as oxidative stress, endoplasmic reticulum (ER) stress and changes in mitochondrial permeability. ER stress triggered by ischemia is an important cause of cell death (6). Despite attempts to improve MSC survival with growth factors, drug pre-treatment, a gene transfection-activated survival pathway and by reducing mitochondrial-mediated apoptosis (7–9), few of these therapies have exerted beneficial effects on ER stress-induced apoptosis.
Glucagon-like peptide-1 (GLP-1) is a peptide secreted from L-cells in the small intestine and the proximal colon. As a cognate receptor for GLP-1, GLP-1 receptor (GLP-1R) is expressed in various types of tissue, such as the brain and the pancreas tissue. Thus, GLP-1 exerts pleiotropic effects, including the enhanced synthesis and release of insulin, enhanced satiety, delayed gastric emptying and increased cellular survival (10). GLP-1 is rapidly cleaved by dipeptidyl peptidase IV (DPPIV) and thus, it has a short half-life. Exendin-4 (ex-4), a 39 amino acid agonist of GLP-1R, has similar biochemical effects to GLP-1; however, it has a longer half-life (11). At present, ex-4 is being used to increase insulin production for the clinical treatment of type 2 diabetes (12). Apart from the insulinotropic effects of ex-4, it has been shown to protect the heart from ischemia-reperfusion injury (13), and it has also been shown to render cells resistant to ischemic-related injury in an experimental model of transient cerebral ischemic damage (14). Previous research has demonstrated that ex-4 attenuates atherosclerotic plaque formation by inhibiting the inflammatory response in macrophages (15). Moreover, ex-4 has been shown to improve the survival of several types of cells, such as β-cells, cardiomyocytes and cholangiocytes (16–18). A growing body of evidence supports the notion that ex-4 plays an important role in the regulation of ER stress, thus exerting cytoprotective effects (16,19). However, to the best of our knowledge, whether ex-4 protects MSCs from ischemia-induced apoptosis and the involvement of ER stress in this process remains unknown.
In consideration of the above-mentioned findings, we hypothesized that ex-4 may confer resistance to apoptosis in MSCs. In this study, we investigated the potential protective effects of ex-4 in rat bone marrow-derived mesenchymal stem cells (BM-MSCs) subjected to oxygen, glucose and serum deprivation (OGD) conditions, as well as the underlying mechanisms.
Materials and methods
Animals and cell culture
This study was approved by the Institutional Animal Care and Use Committee of Harbin Medical University (Harbin, China) and was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (20). Male Sprague Dawley (SD) rats, 2–4 weeks old and weighing 50–60 g, were used in the present study. The pancreases of the rats were isolated and RNA and protein were extracted from the pancreatic tissue. The extraction and culture of the rat MSCs was performed as previously described (9). Briefly, bone marrow was washed out from the tibia and femur marrow cavities of the SD rats, and suspended in sterile phosphate-buffered saline (PBS). Red cells were then lysed and removed, and 5×105 cells were plated in a 25 cm2 flask with F12/Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (both from HyClone, Logan, UT, USA) and 1% penicillin-streptomycin. Following culture for 48 h, the suspended cells and medium were removed, and the adherent MSCs continued to grow in medium that had been refreshed. The MSCs were cultured to 80–90% confluence before passaging. All experiments were performed using MSCs at passages 3–5 and the MSCs were identified by their immunophenotypic characteristics. Briefly, the cells were harvested, washed with PBS and labeled with the following conjugated antibodies: phycoerythrin (PE)-labeled anti-CD45 (553091) and fluorescein isothiocyanate (FITC)-labeled-CD29 (555005), CD44 (561859) and CD34 (560238; all from BD Biosciences, Franklin Lakes, NJ, USA). The labeled cells were detected by flow cytometry and analyzed using FACSDiva software (Becton-Dickinson, San Jose, CA, USA).
Cell treatments
To induce the apoptosis of the BM-MSCs by subjecting them to OGD conditions, the original medium was removed and replaced with glucose- and serum-free medium. The MSCs were then placed in an oxygen free incubator (855-AC; Plas-Labs Inc., Lansing, MI, USA) at 37°C, cultured for 0, 2, 4 and 8 h and then harvested for the analysis of apoptosis. To determine the possible mechanisms responsible for OGD-induced injury, the MSCs were subjected to OGD conditions for 0, 1, 2, 4 and 8 h before being harvesting for further experiments. To determine the optimal concentration of ex-4 (ProSpec-Tany TechnoGene, Ltd., Ness-Ziona, Israel), 10, 50 and 100 nM ex-4 were added to the complete medium 12 h before the cells were subjected to OGD conditions. Cell viability was examined to determine the optimal concentration of ex-4. To examine the anti-apoptotic effects of ex-4, the cells were incubated with ex-4 for 12 h and then subjected to OGD conditions for 4 h. To determine the role of GLP-1R, the cells were incubated with the GLP-1R antagonist, exendin9-39 (ex9-39; 50 nM; Aladdin Reagents (Shanghai) Co., Ltd., Shanghai, China), and ex-4 for 90 min and then subjected to OGD conditions. To determine the involvement of protein kinase A (PKA) in the biological effects of ex-4, the PKA inhibitor, H89 (10 µM; Sigma-Aldrich, St. Louis, MO, USA), was added to complete medium with ex-4, 90 min prior to the cells being subjected to OGD conditions. After being subjected to the experimental treatments, the cells were harvested for use in subsequent experiments.
Gene knockdown of activating transcription factor 4 (ATF-4) and C/EBP homologous protein (CHOP) by small interference RNA (siRNA)
The MSCs were seeded in 6-well plates and cultured in serum-free medium for 24 h, and then transfected with siRNA for rat ATF-4/CHOP and non-target siRNA (scramble siRNA) using X-tremeGENE siRNA transfection reagent (Roche, Mannheim, Germany) according to the manufacturer's instructions. The siRNA sequences for ATF-4 and CHOP were 5′-AUCGAAGUCAAACUCUUUCAGGUCC-3′ and antisense, 5′-GGACCUGAAAGAGUUUGACUUCGAU-3′; and 5′-GGAAGAACUAGGAAACGGA-3′ and antisense, 5′-UCCGUUUCCUAGUUCUUCC-3′, respectively. The siRNAs were dissolved in diethylpryocarbonate (DEPC)-treated water and diluted to 0.2 µM with 250 µl Opti-MEM (obtained from Invitrogen, Carlsbad, CA, USA) for 10 min. A total of 10 µl of X-tremeGENE siRNA transfection reagent (Roche) was also diluted with 250 µl Opti-MEM for 20 min. The siRNA and transfection reagent were then mixed (500 µl) and blended gently for 20 min and added to the cell plate with 2 ml culture medium. The MSCs transfected with the siRNAs were cultured for 48 h and then subjected to OGD conditions as described above. The cells were harvested for reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blot analysis to determine the silencing rate, and for use in subsequent experiments.
MTT assay
The cells were seeded in 96-well plates at a starting density of 1×104 cells/well. After being subjected to the experimental treatments, the cells were washed and incubated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (Sigma-Aldrich) at 37°C for 4 h. The supernatant was removed, and 150 µl dimethyl sulfoxide (DMSO) were added to each well. The absorbance (OD) of the reaction solution was examined at 490 nm. Five separate experiments in triplicate were conducted with different concentrations of ex-4.
Detection of apoptosis by flow cytometric analysis
Apoptosis was assessed by Annexin V-FITC/propidium iodide (PI) (BD Biosciences) double staining and measured by fluorescence-activated cell sorting (FACS). We obtained an Annexin V/PI apoptosis analysis kit and performed the experiment according to the manufacturer's instructions. Briefly, the cells were collected and sorted into assay tubes at a density of 105–5×105 cells/tube. The cells were then stained with Annexin V-FITC and PI for 15 min and detected by FACS with FACSDiva Software (Becton-Dickinson)
Hoechst 33258 staining
The MSCs were seeded in 6-well plates. After being subjected to the experimental treatments, the cells were washed twice with PBS and incubated with Hoechst 33258 staining solution (Beyotime Institute of Biotechnology, Haimen, China) for 30 min at 37°C. Subsequently, the staining solution was removed and the cells were washed twice with cold PBS and images were captured using a fluorescence microscope (DMI4000B; Leica, Wetzlar, Germany). The cells with bright white fluorescent nuclei were considered to be apoptotic cells and those with homogeneous blue fluorescence in the nuclei were considered to be viable cells.
Intracellular cAMP ELISA assay
The MSCs were plated at a density of 5×104 cells in 48-well plates, and after being subjected to the experimental treatments, the cells were collected and lysed by freeze and thaw cycle 5 times. The intracellular cAMP concentrations were measured using a rat cAMP ELISA kit (Jiang Lai Biotechnology, Shanghai, China) according to the manufacturer's instructions. The absorbance (OD) of the reaction solution was detected using a microplate reader (BioTek Instruments, San Jose, CA, USA) at 595 nm.
RT-qPCR
Total RNA was extracted from the MSCs using TRIzol (Invitrogen). The concentration of the RNA was measured by ultraviolet spectrophotometry and 1 µg RNA was reverse transcribed into first-strand cDNA using a Transcriptor First Strand cDNA Synthesis kit (Roche). Quantitative (real-time) (qPCR) was performed using FastStart Universal SYBR-Green Master according to the instructions provided by the manufacturer. The primer sequences were as follows: GLP-1R forward, ACCTGTCGGAGTGCGAAGAGT and reverse, ACAGTGCTCGGAGGATGAAGG; CHOP forward, AGGTCCTGTCCTCAGATGAAAT and reverse, CAGGGTCAAGAGTAGTGAAGGTTT; ATF-4 forward, CCTTCG ACCAGTCGGGTTTG and reverse, CTGTCCCGGAAAAGGCATCC; GAPDH forward, CATCAAGAAGGTGGTGAAGC and reverse, ACCACCCTGTTGCTGTAG. The threshold cycle (Ct) value was detected using an ABI Real Time PCR System (Applied Biosystems, Foster City, CA, USA. The ΔCt value was calculated by subtracting the Ct number of the target gene from that of the housekeeping gene, GAPDH. The fold-change in the transcript level was calculated based on the ΔΔCt method.
Western blot analysis
The MSCs subjected to different experimental treatments were washed with PBS and lysed in RIPA lysis buffer blended with protease and phosphatase inhibitors on ice for 30 min, and subsequently shattered by ultrasound. The cell extracts were centrifuged for 15 min at 12,000 × g and the supernatants were collected. Total protein in the supernatant was quantified using a Bradford protein assay kit (Beyotime Institute of Biotechnology). Proteins (20–30 µg) were separated by SDS-PAGE on a 10% polyacrylamide gel (15% polyacrylamide gel for caspase-3) and then transferred onto methanol-activated PVDF membranes. The membranes were blocked for 1 h in 5% skim milk diluted with TBS (50 mM Tris and 150 mM NaCl) containing 0.1% Tween-20 (TBST) at 37°C and incubated overnight at 4°C with the following primary antibodies: phosphorylated (p-) protein kinase RNA-like endoplasmic reticulum kinase (PERK; 3179), PERK (3192), binding immunoglobulin protein (BIP; 3183), ATF-4 (11815), CHOP (2895), caspase-3 (9665) and Bcl-2 (2870; all from Cell Signaling Technology, Danvers, MA, USA) and GLP-1R (ab39072; Abcam, Cambridge, UK). The membranes were washed 3 times with TBST and then incubated for 1 h with a secondary antibody conjugated with horseradish peroxidase (HRP) (anti-rabbit; sc-25778; Zhongshan Golden Bridge Biotechnology, Beijing, China). The membranes were washed with TBST 3 times. The immune complexes images were developed by ECL in the dark and images were captured using Bio-Rad ChemiDoc XRS equipment, and the protein band density was quantified and analyzed by Quantity One software (both from Bio-Rad, Hercules, CA, USA).
Statistical analysis
Experimental values are expressed as the means ± SD, and the difference between groups was analyzed by one-way ANOVA with Tukey's and Newman Keuls post tests. Statistical analysis was performed by SPSS 19.0 software (IBM Corp., Armonk, NY, USA). P-values <0.05 were considered to indicate a statistically significant difference.
Results
Identification and characterization of BM-MSCs
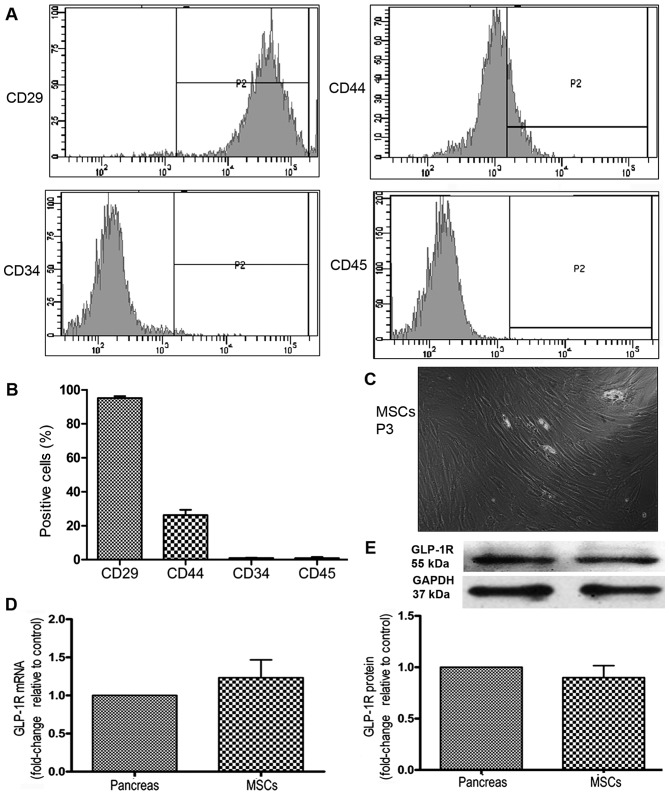
BM-MSCs from passages 3–5 were collected for immunophenotypic identification. The mesenchymal origin markers, CD29 and CD44, were highly expressed, whereas the hematopoietic origin markers, CD45 and CD34, were minimally expressed (Fig. 1A and B). The MSCs were spindle-shaped (Fig. 1C). To examine the effects of the GLP-1R agonist, ex-4, we detected the expression of its specific receptor, GLP-1R, on the MSCs using RT-qPCR and western blot analysis. The results indicated that the MSCs expressed GLP-1R at the mRNA and protein level (Fig. 1D and E).
Figure 1.
Characterization of mesenchymal stem cells (MSCs). MSC cell surface markers were detected by FACS. (A) Representative histograms of immuophenotypic assay for CD29, CD44, CD34 and CD45. (B) Quantitative analysis of cell surface marker expression. (C) Image of MSCs at passage 3 (P3) captured by microscopy at a magnification of x100. (D) The mRNA expression of glucagon-like peptide-1 receptor (GLP-1R) on MSCs was detected by RT-qPCR. Its expression on pancreas served as a positive control. (E) The protein expression of GLP-1R on MSCs was detected by western blot analysis. Its expression on pancreas was served as a positive control.
OGD mimics in vitro ischemic conditions and induces the apoptosis of MSCs
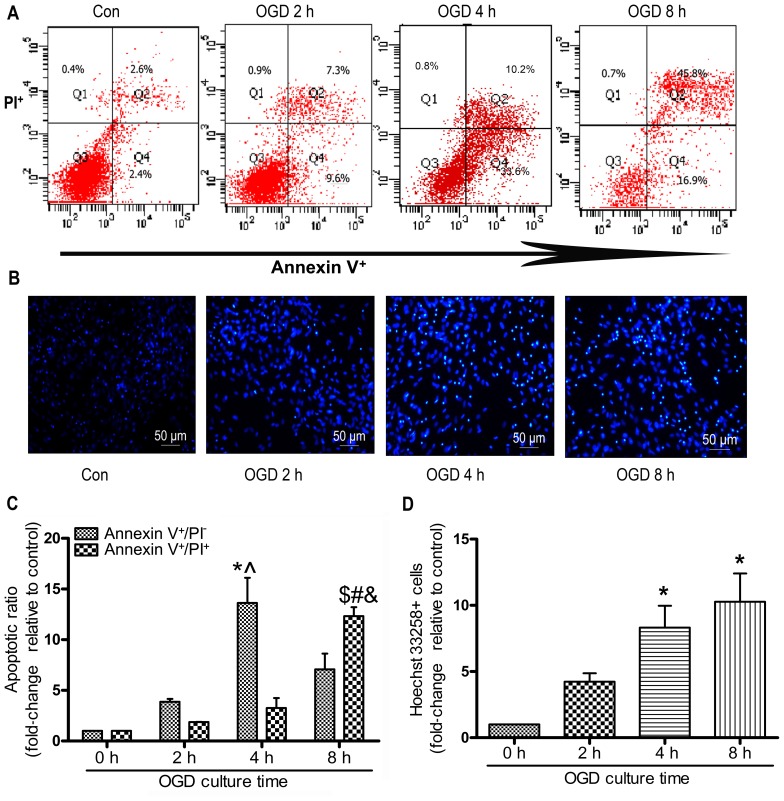
In the present study, the MSCs were subjected to OGD conditions for 0 to 8 h in order to induce typical apoptosis. The apoptotic ratio of the MSCs was detected using Annexin V/PI staining and flow cytometry. As shown in Fig. 2A and C, the early-stage apoptotic ratio within 4 h increased in a time-dependent manner. Subjecting the MSCs to OGD contitions for 4 h resulted in the most notable early-stage apoptotic rate compared with 2 and 8 h of being subjected to OGD contitions (4 h, 13.63±4.28 vs. 2 h 3.86±0.50; 8 h, 7.07±2.69, P<0.05; Fig. 2C). The late-stage apoptotic ratio increased gradually and there was an evident increase in the cells exposed for 8 h to OGD compared with those exposed for 4 or 2 h. The numbers of Hoechst 33258-positive stained cells at 4 and 8 h were significantly increased compared with those at 0 h (4 h, 7.40±0.40; 8 h, 9.20±1.20 vs. 2 h 3.90±0.61; P<0.05; Fig. 2D). However, there was no significant difference observed in the number of Hoechst 33258-positive cells following 4 and 8 h of incubation (P>0.05; Fig. 2D).
Figure 2.
Oxygen and glucose deprivation (OGD) induces the apoptosis of mesenchymal stem cells (MSCs). MSCs were subjected to OGD conditions for the indicated periods of times, and the apoptotic ratio was detected. Apoptosis was assessed by Annexin V/propidium iodide (PI) double staining and FACS. (A) Representative FACS plots. (B) The nuclei of the MSCs were dyed with Hoechst 33258 and representative images of Hoechst 33258-stained cells were obtained under a fluorescence microscope; each experiment was performed 3 times, (C) Quantitative analysis of percentage of early-stage apoptotic cells (Annexin V+/PI−) and late-stage apoptotic (Annexin V+/PI+) cells, each column represents the means ± SD of 3 independent experiments. Annexin V+/PI− cells: *P<0.05 vs. 0 h group; ^P<0.05 vs. 8 h group; Annexin V+/PI+ cells: $P<0.05 vs. 0 h group; #P<0.05 vs. 2 h group; &P<0.05 vs. 4 h group. (D) Quantitative analysis of Hoechst 33258 stained-cells, the cells were shown at magnification of x100, each column represents mean ± SD of 3 independent experiments. *P<0.05 vs. 0 h group.
Exposure to OGD in vitro triggers ER stress in MSCs
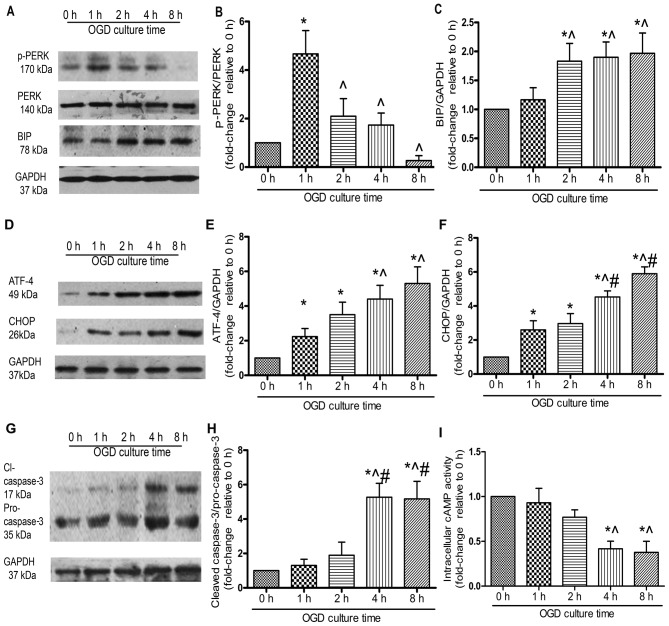
PERK is a critical ER stress sensor, and its activation suggests the involvement of ER stress (21). In the present study, PERK was phosphorylated at 1 h of OGD incubation and this lasted for 4 h (Fig. 3A and B). The protein level of BIP, an ER chaperone, increased significantly following exposure to OGD for 2 h (P<0.05; Fig. 3A and C). ATF-4 protein expression increased gradually. There was a significant elevation at 4 and 8 h compared with that at 1 h (P<0.05; Fig. 3D and E), whereas no marked difference was observed between 4 and 8 h (P>0.05). Similarly, CHOP protein expression at 4 h was significantly increased compared with that at 1 and 2 h, whereas no significant increase was observed between 4 and 8 h (Fig. 3D and F). The cleavage of caspase-3, a typical indicator of cell apoptosis, was significantly upregulated under OGD conditions in a time-dependent manner (Fig. 3G and H). To determine the role of cAMP signaling in the OGD-induced apoptosis of MSCs, we examined the activity of intracellular cAMP, which was significantly decreased under OGD conditions. By 4 h, the activity of intracellular cAMP had decreased by approximately 50%, which suggested that cAMP signaling was abrogated during the OGD-induced apoptosis of MSCs (Fig. 3I).
Figure 3.
Endoplasmic reticulum (ER) stress is involved in the oxygen and glucose deprivation (OGD)-mediated apoptosis of mesenchymal stem cells (MSCs). MSCs were incubated under OGD conditions for the indicated periods of time, and the protein was extracted and quantified by western blot (WB) analysis. All experiments were performed in triplicate. (A) Representative western blots of phosphorylated (p-)PERK, PERK, BIP and the loading control, GAPDH. Relative quantity of (B) p-PERK was estimated as fold-changes relative to PERK, (C) BIP was estimated as fold-changes relative to GAPDH. (D) Representative western blots of ATF-4 and CHOP. Quantitative analysis of (E) ATF-4 and (F) CHOP was estimated as fold-changes relative to GAPDH. (G) Representative western blots of cleaved caspase-3 and pro-caspase-3. (H) The expression of cleaved caspase-3 was estimated as fold-changes relative to pro-caspase-3. (I) MSCs were subjected to OGD conditions for the indicated periods of time and intracellular cAMP activity was assessed by ELISA, each column represents the means ± SD of 3 independent experiments. *P<0.05 vs. 0 h; ^P<0.05 vs. 1 h; #P<0.05 vs. 2 h.
Ex-4 exerts anti-apoptotic effects on MSCs under OGD conditions
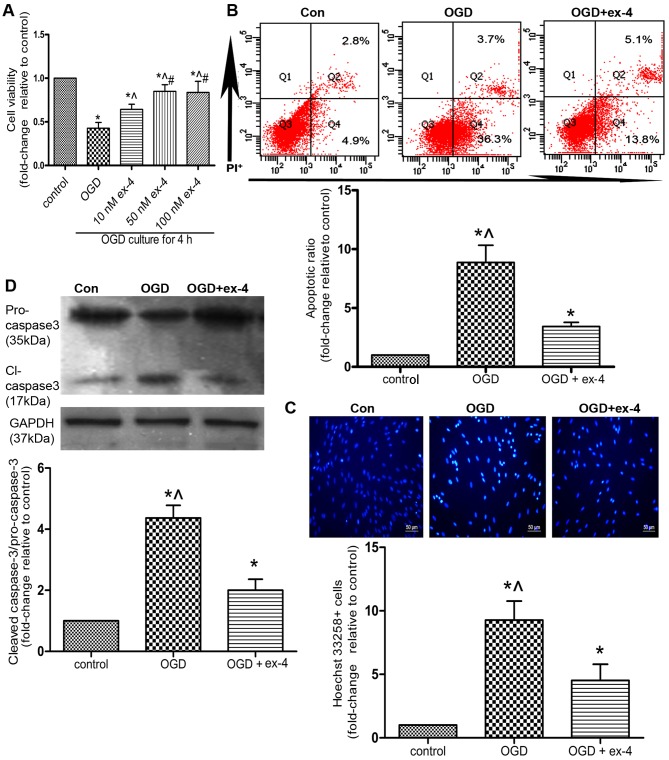
In order to preliminarily evaluate the cytoprotective effects, as well as the corresponding optimal concentration of ex-4 in the MSCs under OGD conditions, an MTT assay was performed on the MSCs pre-treated with 0, 10, 50 and 100 nM of ex-4. As shown in Fig. 4A, pre-treatment of the MSCs with 50 nM ex-4 for 12 h led to the restoration of cell viability by almost 50% compared with the OGD group (cells not treated with ex-4; 0.85±0.04 vs. 0.43±0.04, P<0.05). However, there was no significant difference observed between the 50 nM ex-4- and 100 nM ex-4-treated groups (P>0.05; Fig. 4A). Thus, for subsequent experiments, the MSCs were pre-treated with 50 nM ex-4 in order to evaluate its cytoprotective effects.
Figure 4.
Exendin-4 (ex-4) protects mesenchymal stem cells (MSCs) from oxygen and glucose deprivation (OGD)-induced apoptosis. MSCs were pre-treated with ex-4 for 12 h and subsequently incubated under OGD conditions for 4 h. All experiments were conducted in triplicate. (A) Cell viability was detected by an MTT assay. *P<0.05 vs. control group; ^P<0.05 vs. OGD group; #P<0.05 vs. 10 nM ex-4 group. Apoptosis was detected by Annexin V/propidium iodide (PI) staining and FACS. (B) Representative FACS plots and quantitative analysis of the apoptotic ratio. The apoptotic ratio was the percentage summation of early-stage apoptotic cells (Q4) and late-stage apoptotic (Q2) cells. (C) Representative images of Hoechst 33258-stained cells and quantitative analysis of the ratio of Hoechst 33258-positive cells. The cells were shown at magnification of x100. (D) The expression of cleaved caspase-3 and pro-caspase-3 detected by western blot analysis. For (B–D) *P<0.05 vs. control group; ^P<0.05 vs. OGD + ex-4 group.
The apoptotic ratio of the MSCs was detected by Annexin V/PI staining and flow cytometry. As indicated in Fig 4B, pre-treatment with ex-4 reduced the apoptotic ratio by 61.3% compared with that in the OGD group (3.43±0.35 vs. 8.87±1.46, P<0.05). In a parallel experiment, the apoptosis of the MSCs was assessed by Hoechst 33258 staining. The number of Hoechst 33258-positive cells was also reduced by 51.6% in the OGD + ex-4 group compared with that in the OGD group (4.52±1.28 vs. 9.27±1.50, P<0.05; Fig. 4C). Moreover, it was demonstrated that the levels of cleaved caspase-3 were markedly reduced in the OGD + ex-4 group compared with those in the OGD group (2.00±0.36 vs. 4.37±0.42, P<0.05; Fig. 4D). Taken together, these findings indicated that ex-4 exerted anti-apoptotic effects on the MSCs under OGD conditions.
Ex-4 protects MSCs from OGD-induced apoptosis by decreasing the ATF-4 and CHOP levels and by activating the GLP-1R/cAMP/PKA pathway
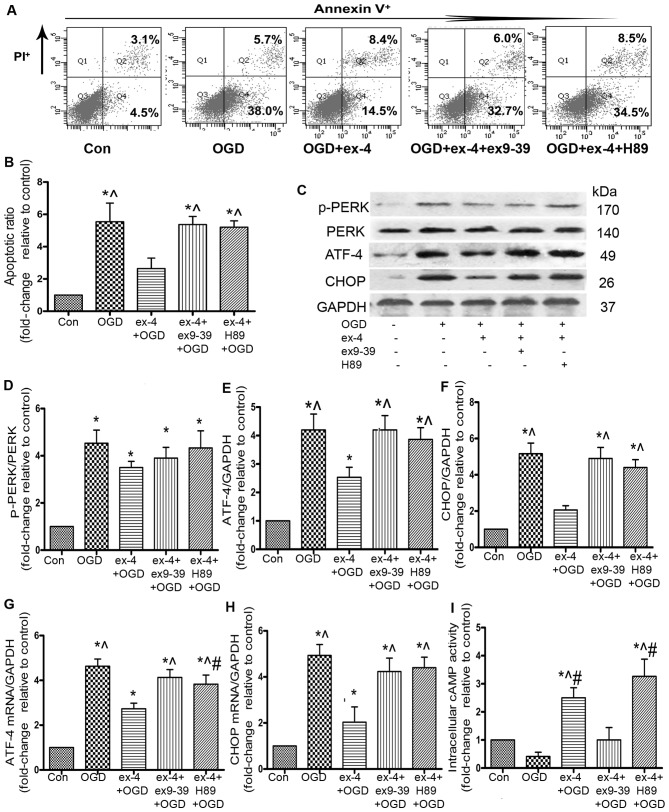
It has been demonstrated that ATF-4 and CHOP are critical factors that contribute to cell apoptosis induced by ER stress (22). In the present study, the ATF-4 mRNA levels decreased by 41.3% (2.73±0.25 vs. 4.63±0.32, P<0.05; Fig. 5G) and the CHOP mRNA levels decreased by 59.2% in the ex-4 + OGD group compared with the OGD group (2.03±0.67 vs. 4.93±0.47, P<0.05; Fig. 5H). To further confirm the inhibition of the ATF-4/CHOP pathway, the ATF-4 and CHOP protein levels were examined by western blot analysis; ex-4 significantly reduced the protein expression of ATF-4 by 39.8% compared with the OGD group (2.53±0.61 vs. 4.20±0.96, P<0.05; Fig. 5E), and CHOP protein expression decreased by 60% compared with the OGD group (2.07±0.40 vs. 5.17±1.00, P<0.05; Fig. 5F). There was no significant difference observed in the p-PERK protein levels between the ex-4 + OGD and the OGD groups (P>0.05; Fig. 5D).
Figure 5.
Exendin (ex)-4 reduces apoptosis of mesenchymal stem cells (MSCs) by activating the glucagon-like peptide-1 receptor (GLP-1R)/PKA/cAMP pathway and inhibiting the activation of the ATF-4/CHOP pathway. MSCs were pre-treated with ex-4, the GLP-1R antagonist, ex-9-39 (50 nM), or the PKA inhibitor, H89 (10 µM), prior to exposure to oxygen and glucose deprivation (OGD) for 4 h. (A) The apoptosis of MSCs were detected by FACS. (B) The apoptotic ratio of MSCs was quantified. (C) The protein expression of phosphorylated (p-)PERK, PERK, ATF-4 and CHOP was detected by western blot analysis. (D) The expression of p-PERK was estimated as fold-changes relative to PERK. The expression of (E) ATF-4 and (F) CHOP was estimated as fold-changes relative to GAPDH. The mRNA expression of (G) ATF-4 and (H) CHOP was detected by RT-qPCR. All experiments were repeated 3 times. *P<0.05 vs. control group; ^P<0.05 vs. ex-4 + OGD group; #P<0.05 vs. OGD group. (I) Intracellular cAMP activity was detected by ELISA assay, each column represents the means ± SD from 3 independent experiments, *P<0.05 vs. control group; ^P<0.05 vs. OGD group; #P<0.05 vs. ex-4 + ex9-39 + OGD group.
To examine the role of GLP-1R in the MSCs pre-treated with ex-4, the MSCs were incubated with the GLP-1R antagonist, ex9-39, as well as ex-4, prior to incubation under OGD conditions. The apoptotic rate was much higher in the ex-4 + ex9-39 + OGD group than that in the ex-4 + OGD group (5.37±0.50 vs. 2.64±0.65, P<0.05; Fig. 5A and B). Following incubation with ex9-39, the abrogation of ATF-4 (2.53±0.61 vs. 4.20±0.87, P<0.05; Fig. 5E) and CHOP protein expression was inhibited (2.07±0.40 vs. 4.90±1.06, P<0.05; Fig. 5F). The mRNA expression of ATF-4 and CHOP was also significantly higher in the ex-4 + ex9-39 + OGD group than that in the ex-4 + OGD group (P<0.05; Fig. 5G and H).
The cAMP-dependent PKA pathway has been found to play a role in cell survival (23). Thus, to examine the involvement of the cAMP/PKA pathway in the anti-apoptotic effects of ex-4, we conducted an intracellular cAMP activity ELISA, which showed that the cAMP activity in the ex-4 + OGD group was markedly increased compared with that in the OGD and ex9-39 + ex-4 + OGD group (ex-4 2.50±0.36 vs. OGD 0.42±0.18, ex9-39 1.00±0.44, P<0.05; Fig. 5I), which suggested that ex-4 enhanced the activity of cAMP. In order to examine the role of PKA in the cytoprotective effects of ex-4, the MSCs were pre-incubated with H89. FACS analysis revealed that the apoptotic ratio in the ex-4 + H89 + OGD group was significantly increased compared with that in the ex-4 + OGD group (ex-4, 2.64±0.65 vs. H89, 5.20±0.39, P<0.05; Fig. 5B), which suggests that PKA plays a major role in the anti-apoptotic effects of ex-4. As regards the role of PKA in the inhibitory effects of ex-4 on ATF-4/CHOP, the results revealed that there was no marked difference between the OGD and the ex-4 + H89 + OGD groups in the ATF-4/CHOP protein levels, as well as in the CHOP mRNA level (P>0.05; Fig. 5E, F and H), whereas there were prominent differences in the mRNA and protein levels of ATF-4 and CHOP between the ex-4 + OGD group and the ex-4 + H89 + OGD group (P<0.05; Fig. 5E–H). Taken together, these findings suggest that the activation of PKA may participate in the abrogation of ATF-4/CHOP levels by ex-4.
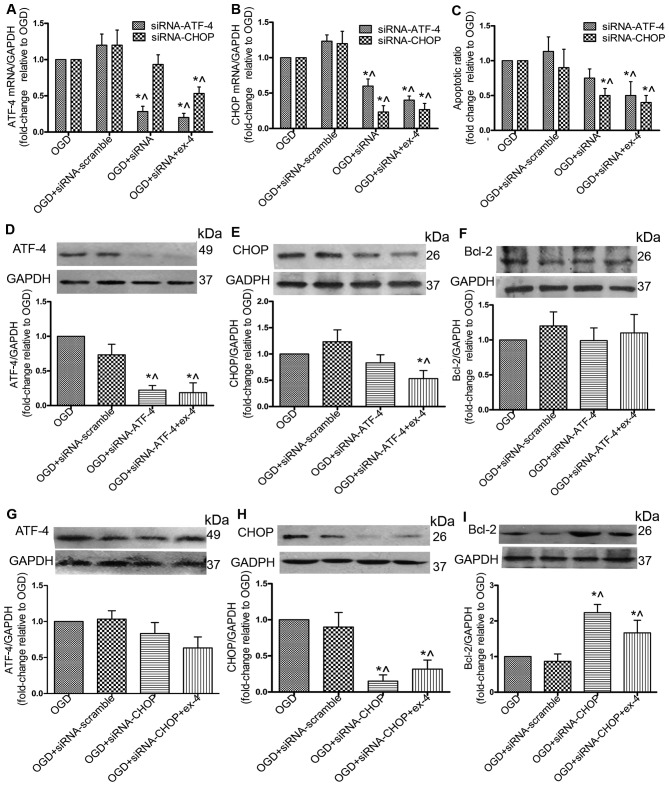
siRNA against CHOP (siRNA-CHOP) protects MSCs by upregulating Bcl-2 and does not impair the cytoprotective effects of ex-4
To further explore the role of the ATF-4/CHOP pathway in the anti-apoptotic effects mediated by ex-4, the siRNA knockdown technique was adopted to reduce the expression of ATF-4 and CHOP. By silencing ATF-4, the mRNA expression of ATF-4 was reduced by 72% (P<0.05; Fig. 6A), and the protein expression was reduced by 73% (P<0.05; Fig. 6D); however, no significant difference in the apoptotic ratio and Bcl-2 protein expression was observed between the OGD + siRNA-ATF-4 group and the OGD group (P>0.05; Fig. 6C and F). Treatment with Ex-4 combined with siRNA-ATF-4 significantly decreased the apoptotic ratio (Fig. 6C) and the protein expression of CHOP (Fig. 6E). By silencing CHOP, its protein expression was reduced by 87% (Fig. 6H) and the apoptotic ratio was decreased by 50% (Fig. 6C). There was no significant change in the ATF-4 protein levels (Fig. 6G); however, Bcl-2 protein expression was increased 1.23-fold (Fig. 6I). With ex-4 + siRNA-CHOP treatment, the apoptotic ratio was decreased (P<0.05; Fig. 6C), and in addition, the Bcl-2 protein levels were significantly upregulated compared with the OGD group (P<0.05; Fig. 6I). These results suggested that transfection of the cells with siRNA-CHOP reduced the apoptotic rate in the MSCs without impairing the anti-apoptotic effects of ex-4.
Figure 6.
Small interfering RNA (siRNA) gene knockdown of CHOP increases the Bcl-2 protein level and reduces apoptosis without however, impairing the anti-apoptotic effect of exendin-4 (ex-4). Mesenchymal stem cells (MSCs) were transfected with siRNA scramble/siRNA ATF-4/siRNA CHOP or treated with ex-4 prior to incubation under oxygen and glucose deprivation (OGD) conditions. The mRNA level of (A) ATF-4 and (B) CHOP was detected by RT-qPCR. (C) The apoptotic ratio was analyzed by FACS. MSCs were transfected with siRNA scramble/siRNA ATF-4 or treated with ex-4 prior to incubation under OGD conditions and the protein expression of (D) ATF-4, (E) CHOP and (F) Bcl-2 was detected by western blot analysis. MSCs were transfected with siRNA scramble/siRNA CHOP or treated with ex-4 prior to incubation under OGD conditions and the protein expression of (G) ATF-4, (H) CHOP and (I) Bcl-2 was detected by WB analysis. All experiments were repeated 3 times. *P<0.05 vs. OGD group; ^P<0.05 vs. OGD + siRNA-scramble.
Discussion
BM-MSCs originate from the mesodermal germ layer and perform a supportive function in the stroma, as well as giving rise to cells of multiple cell lineages, including adipocytes and osteocytes. Evidence suggests that MSCs possess the ability to differentiate into cardiomyocytes and to secrete a wide array of cytokines and growth factors to suppress the inflammatory response, inhibit fibrosis and enhance angiogenesis in the infarcted myocardium (24). These findings provide a solid theoretical basis for the application of MSC engraftment as a therapy in AMI; however, MSCs do not in fact survive for long following transplantation, which may explain the negligible effect on cardiac function one year after MSC engraftment (25). Based on findings from a bioluminescence imaging study by van der Bogt et al, all engrafted MSCs had died by week 6 (26). In addition, Mangi et al observed robust cell death early following transplantation after transfecting MSCs with the pro-survival gene, Akt-1 (8). The ischemic microenvironment in the infarcted heart proves hostile to engrafted MSCs, resulting in decreased cell survival. To mimic the ischemic milieu in vitro, hypoxia and serum deprivation (H/SD) is a commonly used model, and our group previously found that the H/SD model induced the apoptosis of approximately 25% of MSCs for up to 24 h (7). As glucose is an indispensible energy nutrient for cells and is found in short supply in the ischemic context, the OGD model was adopted in the present study and exposure to OGD induced the apoptosis of approximately 40% of the MSCs within 4 h. In comparison, the OGD model represents a more time-saving and aggressive approach to mediate MSC death in vitro.
To date, studies aiming to find ways to protect MSCs from apoptosis have focused on maintaining the integrity of the mitochondria or activating survival signaling pathways (7). Few studies have highlighted the importance of ER stress induced by ischemia (27). As a matter of fact, ER stress plays a pivotal role in the pathophysiological mechanisms of AMI and ischemia-induced apoptosis (6). The ER is an organelle involved in protein folding, calcium homeostasis and lipid biosynthesis. Various factors that interfere with ER function lead to the accumulation of misfolded or unfolded proteins, including oxidative stress, ischemia and disturbances in calcium homeostasis. Okada et al demonstrated that ER stress-initiated apoptosis occurred in cardiomocytes in a mouse model of aortic constriction, and found that suppressing the ER stress pathway may diminish the death of cardiomocytes (28). Additionally, it was suggested to be the main mechanism responsible for the apoptosis of various types of cells in models of ischemia in vivo or in vitro (29,30). In line with these results, the present study demonstrated that the levels of p-PERK/BIP/ATF-4/CHOP markedly increased and those of the apoptotic indicator, cleaved caspase-3, were upregulated, which strongly suggested that excessive and prolonged ER stress is an important mechanism responsible for the apoptosis of MSCs in the setting of ischemia. ER stress serves as a predominant mechanism and a therapeutic target with which to improve the survival of both cardiomocytes and MSCs in AMI.
Ex-4, a long-acting agonist of GLP-1, is known to regulate the perturbation of ER stress. As a GLP-1 agonist, ex-4 depends on GLP-1R to play its physiological role, and this study demonstrated that GLP-1R was expressed on rat MSCs and played a key role in mediating the anti-apoptotic effects of ex-4. Tsunekawa et al (31) observed that ex-4 distinctly decreased the levels of the ER stress-related molecules, BIP and CHOP, in β-cell-specific calmodulin-overexpressing mice and reduced the apoptosis of β-cells. In agreement with these findings, the present study found that ex-4 markedly decreased the apoptosis of MSCs by inhibiting the activation of the ATF-4/CHOP pathway. Furthermore, in a rat model of myocardial infarction, pre-conditioning with ex-4 was shown to improve the adhesion and therapeutic efficacy of transplanted adipose-derived stem cell transplantation therapy (32). In a previous study, the post-MI delivery of encapsulated GLP-1 MSCs that had been genetically modified increased angiogenesis and attenuated fibrosis than treatment with MSCs alone (33). Moreover, ex-4 aids in the survival of cardiomyocytes and endothelial cells, and the pro-inflammatory factors in the infacted myocardium were significantly decreased by ex-4 infiltration (15,16,34). Taken together, the findings from previous studies and the present study indicate that ex-4 is a promising candidate for optimizing MSC therapy for AMI.
The ATF-4/CHOP pathway is an important branch of ER stress, and the present study demonstrated that ex-4 reduced the OGD-induced apoptosis of MSCs by suppressing the ATF-4/CHOP pathway. CHOP serves as a pivotal stimulus for cell death, and it is mainly induced by ATF-4 (35). The present study found that CHOP-siRNA significantly decreased the apoptosis of MSCs under OGD conditions. In line with these results, a previous study by Zinszner et al (36) demonstrated that embryonic fibroblasts derived from CHOP−/− mice exhibited significantly less apoptosis. CHOP mediated the transcription of various types of apoptotic-related molecules, including Bcl-2, GADD34 and ER oxidoreductin 1 (ERO1) (37). Bcl-2 promotes cell survival by restoring the mitochondrial potential, and the present study indicated that the Bcl-2 protein levels were significantly increased following transfection with siRNA-CHOP and ex-4 preconditioning. This implied that Bcl-2 participated in the anti-apoptotic effects of ex-4. Moreover, CHOP activated GADD34 to initiate or enhance apoptosis by increasing protein synthesis, which resulted in the aggravation of ER stress. By activating ERO1, CHOP promoted the oxidizing state in the ER and therefore increased the aggregation of hostile elements leading to cell death (37). Thus, the downregulation of CHOP in MSCs may be the principal mechanism responsible for the cytoprotective effects of ex-4.
The cAMP-dependent PKA pathway plays a role in the promotion of cell proliferation and in the inhibition of apoptosis (23). The signal transduction to ER by ex-4 has been reported to be associated with the cAMP/PKA pathway in β-cells (38). In the present study, the activity of intracellular cAMP was impaired by OGD, and restored by ex-4. The protective effects of ex-4 were notably reversed by H89, whereas the attenuation of ATF-4 and CHOP levels by ex-4 was partly restored. Thus, this study strongly suggested that the cAMP-dependent PKA pathway may also participate in the anti-apoptotic effects of ex-4 under OGD conditions.
In conclusion, the present study demonstrates that ex-4 confers resistance to OGD-mediated apoptosis in BM-MSCs, and the possible mechanisms responsible for these effects involve the activation of the GLP-1R/cAMP/PKA pathway and the attenuation of ER stress. These findings highlight ER stress as a pivotal target for protecting MSCs from ischemia and provide evidence of the cytoprotective effects of ex-4. Together with the reported protective effects against ischemia or ischemia-reperfusion injury in cardiomyocytes, ex-4 may represent a therapeutic agent with the potential to optimize MSC therapy in AMI.
Acknowledgments
We would like to thank Dr Hulun Li for his guidance with cell culture and treatment, and Dr Wei Liu for her expert assistance with PCR and western blot analysis. Dr Hulun Li and Dr Wei Liu are members of the Key Laboratory of Myocardial Ischemia Mechanism and Treatment (Harbin Medical University), Ministry of Education. The present study was supported by grants from the National Natural Science Foundation of China (grant nos. 81330033 and 81300201) and a grant from the Key Laboratory of Myocardial Ischemia, Harbin Medical University, Ministry of Education (no. KF201408).
References
- 1.Boersma E, Mercado N, Poldermans D, Gardien M, Vos J, Simoons ML. Acute myocardial infarction. 2003;361:847–858. doi: 10.1016/S0140-6736(03)12712-2. [DOI] [PubMed] [Google Scholar]
- 2.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 3.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 4.Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS, Wold LE, Kloner RA. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112:214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 5.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 6.Minamino T, Kitakaze M. ER stress in cardiovascular disease. J Mol Cell Cardiol. 2010;48:1105–1110. doi: 10.1016/j.yjmcc.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Hou M, Liu J, Liu F, Liu K, Yu B. C1q tumor necrosis factor-related protein-3 protects mesenchymal stem cells against hypoxia- and serum deprivation-induced apoptosis through the phosphoinositide 3-kinase/Akt pathway. Int J Mol Med. 2014;33:97–104. doi: 10.3892/ijmm.2013.1550. [DOI] [PubMed] [Google Scholar]
- 8.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 9.Hou M, Cui J, Liu J, Liu F, Jiang R, Liu K, Wang Y, Yin L, Liu W, Yu B. Angiopoietin-like 4 confers resistance to hypoxia/serum deprivation-induced apoptosis through PI3K/Akt and ERK1/2 signaling pathways in mesenchymal stem cells. PLoS One. 2014;9:e85808. doi: 10.1371/journal.pone.0085808. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Willms B, Werner J, Holst JJ, Orskov C, Creutzfeldt W, Nauck MA. Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon-like peptide-1 (GLP-1)-(7-36) amide in type 2 (noninsulin-dependent) diabetic patients. J Clin Endocrinol Metab. 1996;81:327–332. doi: 10.1210/jcem.81.1.8550773. [DOI] [PubMed] [Google Scholar]
- 11.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270–2276. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 12.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients withtype 2 diabetes. Diabetes Care. 2005;28:1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 13.Sonne DP, Engstrøm T, Treiman M. Protective effects of GLP-1 analogues exendin-4 and GLP-1(9-36) amide against ischemia-reperfusion injury in rat heart. Regul Pept. 2008;146:243–249. doi: 10.1016/j.regpep.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Lee CH, Yan B, Yoo KY, Choi JH, Kwon SH, Her S, Sohn Y, Hwang IK, Cho JH, Kim YM, Won MH. Ischemia-induced changes in glucagon-like peptide-1 receptor and neuroprotective effect of its agonist, exendin-4, in experimental transient cerebral ischemia. J Neurosci Res. 2011;89:1103–1113. doi: 10.1002/jnr.22596. [DOI] [PubMed] [Google Scholar]
- 15.Arakawa M, Mita T, Azuma K, Ebato C, Goto H, Nomiyama T, Fujitani Y, Hirose T, Kawamori R, Watada H. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes. 2010;59:1030–1037. doi: 10.2337/db09-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Younce CW, Burmeister MA, Ayala JE. Exendin-4 attenuates high glucose-induced cardiomyocyte apoptosis via inhibition of endoplasmic reticulum stress and activation of SERCA2a. Am J Physiol Cell Physiol. 2013;304:C508–C518. doi: 10.1152/ajpcell.00248.2012. [DOI] [PubMed] [Google Scholar]
- 17.Marzioni M, Alpini G, Saccomanno S, Candelaresi C, Venter J, Rychlicki C, Fava G, Francis H, Trozzi L, Benedetti A. Exendin-4, a glucagon-like peptide 1 receptor agonist, protects cholangiocytes from apoptosis. Gut. 2009;58:990–997. doi: 10.1136/gut.2008.150870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferdaoussi M, Abdelli S, Yang J-Y, Cornu M, Niederhauser G, Favre D, Widmann C, Regazzi R, Thorens B, Waeber G, Abderrahmani A. Exendin-4 protects β-cells from interleukin-1 β-induced apoptosis by interfering with the c-Jun NH2-terminal kinase pathway. Diabetes. 2008;57:1205–1215. doi: 10.2337/db07-1214. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Hong SW, Park SE, Rhee EJ, Park CY, Oh KW, Park SW, Lee WY. Exendin-4 attenuates endoplasmic reticulum stress through a SIRT1-dependent mechanism. Cell Stress Chaperones. 2014;19:649–656. doi: 10.1007/s12192-013-0490-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark JD, Gebhart GF, Gonder JC, Keeling ME, Kohn DF. The 1996 guide for the care and use of laboratory animals. ILAR J. 1997;38:41–48. doi: 10.1093/ilar.38.1.41. [DOI] [PubMed] [Google Scholar]
- 21.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 22.Cao J, Dai D-L, Yao L, Yu H-H, Ning B, Zhang Q, Chen J, Cheng WH, Shen W, Yang ZX. Saturated fatty acid induction of endoplasmic reticulum stress and apoptosis in human liver cells via the PERK/ATF4/CHOP signaling pathway. Mol Cell Biochem. 2012;364:115–129. doi: 10.1007/s11010-011-1211-9. [DOI] [PubMed] [Google Scholar]
- 23.Drucker DJ. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol. 2003;17:161–171. doi: 10.1210/me.2002-0306. [DOI] [PubMed] [Google Scholar]
- 24.Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J, Vanderheyden M, Kim HS, Kang HJ, Strauer BE, Vetrovec GW. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761–1767. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 26.van der Bogt KE, Schrepfer S, Yu J, Sheikh AY, Hoyt G, Govaert JA, Velotta JB, Contag CH, Robbins RC, Wu JC. Comparison of transplantation of adipose tissue- and bone marrow-derived mesenchymal stem cells in the infarcted heart. Transplantation. 2009;87:642–652. doi: 10.1097/TP.0b013e31819609d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szegezdi E, Duffy A, O'Mahoney ME, Logue SE, Mylotte LA, O'brien T, Samali A. ER stress contributes to ischemia-induced cardiomyocyte apoptosis. Biochem Biophys Res Commun. 2006;349:1406–1411. doi: 10.1016/j.bbrc.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani T, et al. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation. 2004;110:705–712. doi: 10.1161/01.CIR.0000137836.95625.D4. [DOI] [PubMed] [Google Scholar]
- 29.Hillion JA, Takahashi K, Maric D, Ruetzler C, Barker JL, Hallenbeck JM. Development of an ischemic tolerance model in a PC12 cell line. J Cereb Blood Flow Metab. 2005;25:154–162. doi: 10.1038/sj.jcbfm.9600003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badiola N, Penas C, Miñano-Molina A, Barneda-Zahonero B, Fadó R, Sánchez-Opazo G, Comella JX, Sabriá J, Zhu C, Blomgren K, et al. Induction of ER stress in response to oxygen-glucose deprivation of cortical cultures involves the activation of the PERK and IRE-1 pathways and of caspase-12. Cell Death Dis. 2011;2:e149. doi: 10.1038/cddis.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsunekawa S, Yamamoto N, Tsukamoto K, Itoh Y, Kaneko Y, Kimura T, Ariyoshi Y, Miura Y, Oiso Y, Niki I. Protection of pancreatic beta-cells by exendin-4 may involve the reduction of endoplasmic reticulum stress; in vivo and in vitro studies. J Endocrinol. 2007;193:65–74. doi: 10.1677/JOE-06-0148. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Wang H, Wang Y, Yin Y, Wang L, Liu Z, Yang J, Chen Y, Wang C. Exendin-4 pretreated adipose derived stem cells are resistant to oxidative stress and improve cardiac performance via enhanced adhesion in the infarcted heart. PLoS One. 2014;9:e99756. doi: 10.1371/journal.pone.0099756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright EJ, Farrell KA, Malik N, Kassem M, Lewis AL, Wallrapp C, Holt CM. Encapsulated glucagon-like peptide-1-producing mesenchymal stem cells have a beneficial effect on failing pig hearts. Stem Cells Transl Med. 2012;1:759–769. doi: 10.5966/sctm.2012-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Favaro E, Granata R, Miceli I, Baragli A, Settanni F, Cavallo Perin P, Ghigo E, Camussi G, Zanone MM. The ghrelin gene products and exendin-4 promote survival of human pancreatic islet endothelial cells inhyperglycaemic conditions, through phosphoinositide 3-kinase/Akt, extracellular signal-related kinase (ERK)1/2 and cAMP/protein kinase A (PKA) signalling pathways. Diabetologia. 2012;55:1058–1070. doi: 10.1007/s00125-011-2423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 36.Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cunha DA, Ladrière L, Ortis F, Igoillo-Esteve M, Gurzov EN, Lupi R, Marchetti P, Eizirik DL, Cnop M. Glucagon-like peptide-1 agonists protect pancreatic β-cells from lipotoxic endoplasmic reticulum stress through upregulation of BiP and JunB. Diabetes. 2009;58:2851–2862. doi: 10.2337/db09-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]