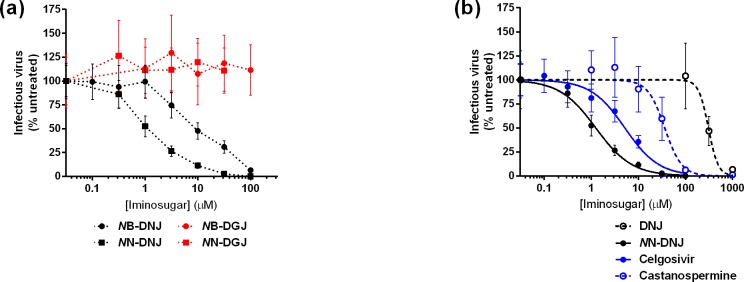
Fig 2. In vitro evaluation of iminosugar antiviral efficacy against DENV.
MDMΦs were infected with DENV (serotype 2, MOI = 1) and treated with a titration of iminosugars for 48 hours. Infectious virus titer was determined by plaque assay using LLC-MK2 (monkey kidney) cells. Counts were normalised to 100 percent for untreated samples. (a) DNJ-derived iminosugars (black) had antiviral efficacy with EC50s between 1.2 and 10.6 μM, whereas DGJ-derived iminosugars (red) were not effective up to the maximum non-toxic dose tested. (b) Parent compounds DNJ and castanospermine were compared to derivatives NN-DNJ and celgosivir, respectively. A four-parameter logistic nonlinear regression curve was determined to calculate EC50 values. Enhancement of antiviral activity by derivatization for NN-DNJ was 246 fold and for celgosivir 7 fold. Each donor was treated in triplicate, and plaque assays were conducted in triplicate on each sample obtained. A minimum of three donors were tested for each compound. Data are presented as mean ± SD.