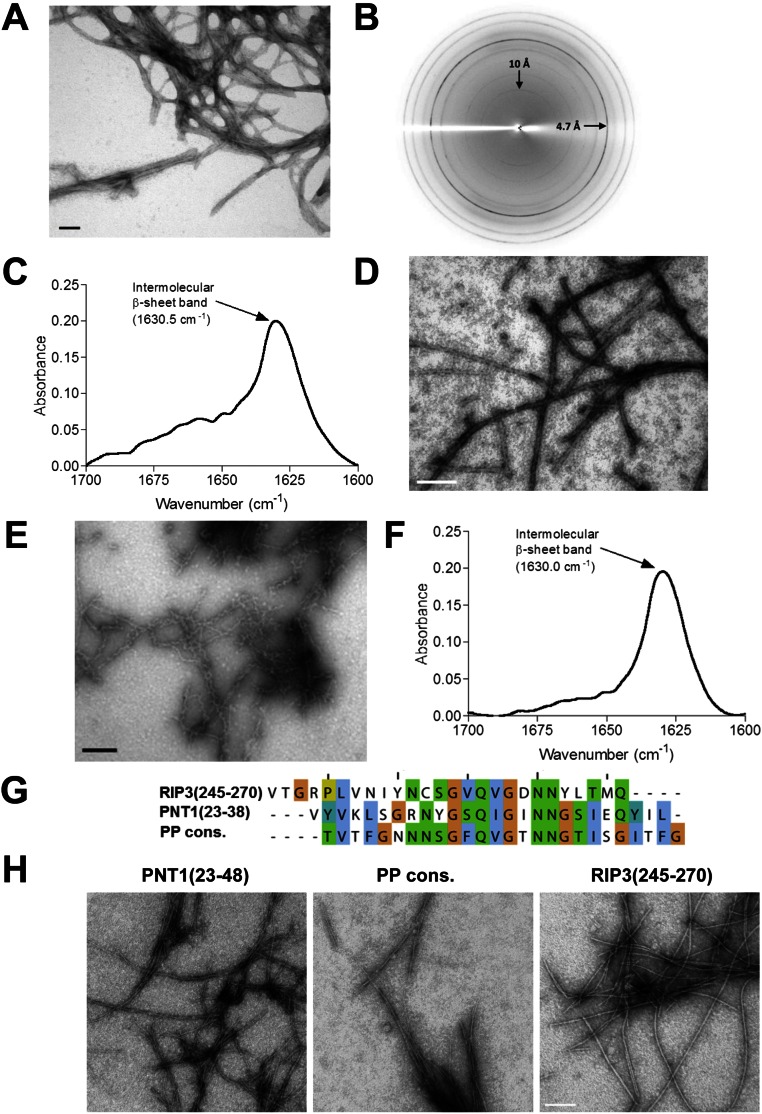
Fig. S4.
HELLP(215-278), SBP(219-280), and PNT1(23-38) form amyloid fibrils. (A) Electron micrograph of negatively stained HELLP(215-278) fibrils. (Scale bar: 30 nm.) (B) X-ray diffraction of HELLP(215-278) fibrils. Note the major diffraction at 4.7 Å. (C) FTIR analysis of HELLP(215-278) fibrils showing a major band at 1,630 cm−1 indicative of the presence of parallel stacked β-sheets. (D) Electron micrograph of HELLP(215-278) fibrils formed in 8 M urea. (Scale bar: 100 nm.) (E) Electron micrograph of negatively stained SBP(219-280) fibrils. (Scale bar: 30 nm.) (F) FTIR analysis of SBP(219-280) fibrils showing a major band at 1,630 cm−1. (G) Sequence and alignment of three 26-aa peptides related to the PP motif and analyzed by EM. (H) Electron micrograph of the assemblies formed by the synthetic peptide as given in E. (Scale bar: 100 nm.)