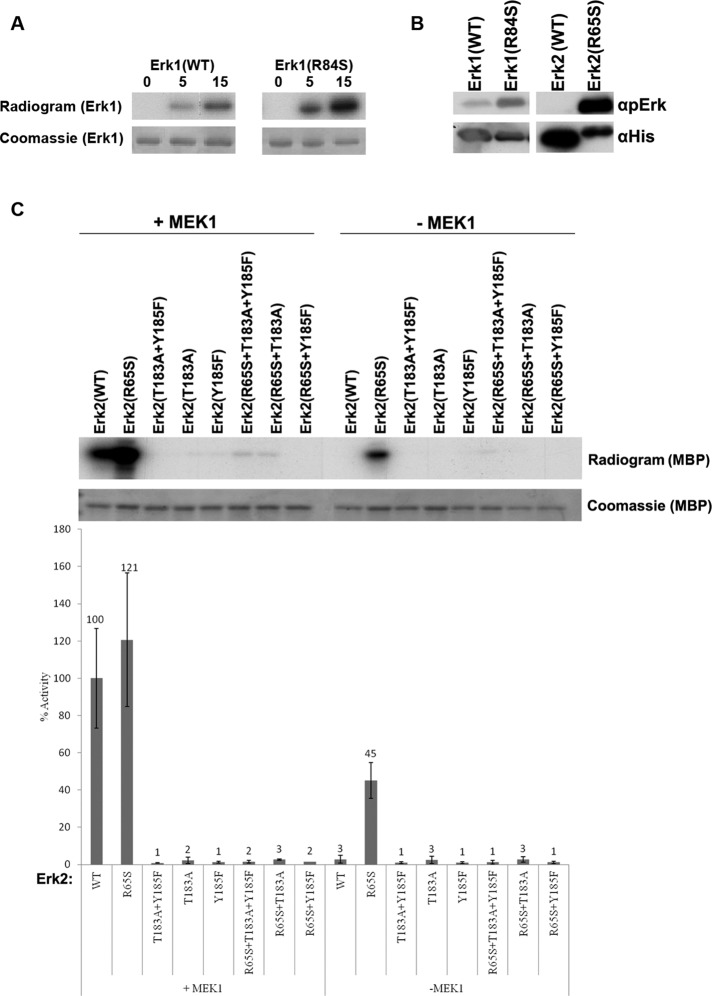
FIGURE 5:
Erk1(R84S) and Erk2(R65S) are capable of efficient autophosphorylation at the TEY motif. (A) Autophosphorylation capabilities of the purified Erks were assessed by incubating the proteins in a kinase assay mixture with [γ-32P]ATP and no other substrate. Reactions were terminated at the indicated time points, separated via SDS–PAGE, stained with Coomassie brilliant blue, and exposed to x-ray film. (B) Autophosphorylation of the active variants occurs at the TEY motif. The indicated proteins were subjected to Western blot analysis using antibodies that react with Erk1/2 proteins phosphorylated at their TEY motif (αpErk) or with antibodies that react with the polyhistidine tag (αHis). (C) Intact TEY motif is essential for catalytic activity of Erk2(R65S) toward the substrate MBP. Catalytic activity of purified recombinant Erks carrying mutations at the TEY motif was analyzed with or without preincubation with active MEK1, using [γ-32p]ATP and MBP as substrates. Reaction mixtures were spotted on filter papers and quantified. Activity of MEK1-activated Erk2(WT) was defined as 100%. In parallel, a sample from each reaction was subjected to SDS–PAGE, stained with Coomassie brilliant blue, and exposed to x-ray film.