FIGURE 1:
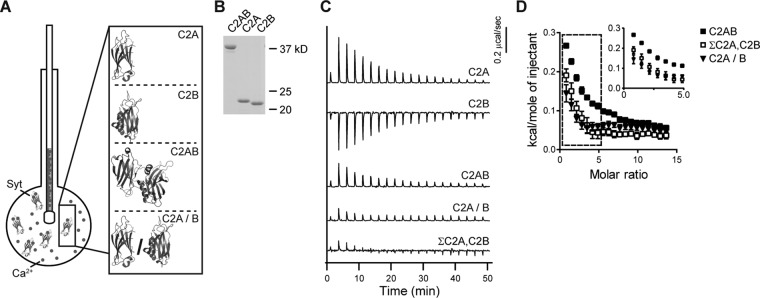
The Ca2+-binding activity of syt-1 depends on tethering its tandem C2 domains together. (A) Schematic illustrating ITC experiments in which Ca2+ is injected into a sample cell containing fragments of syt-1 (structures from Fuson et al., 2007). (B) A representative SDS–PAGE gel of the proteins (0.6 μg) used in the ITC experiments. (C) Heat of binding was measured for equal molar amounts of isolated C2A, isolated C2B, tethered C2AB, or the severed C2 domains, denoted C2A/B. For additional comparison, the traces obtained using isolated C2A and C2B were summed (ΣC2A,C2B). Shown are representative traces; n ≥ 3. (D) Isotherms of ΣC2A,C2B and C2A/B are significantly different from tethered C2AB. The inset shows the first seven data points on an expanded scale; error bars indicate SEM. Thermodynamic values are provided in Table 1.