FIGURE 2:
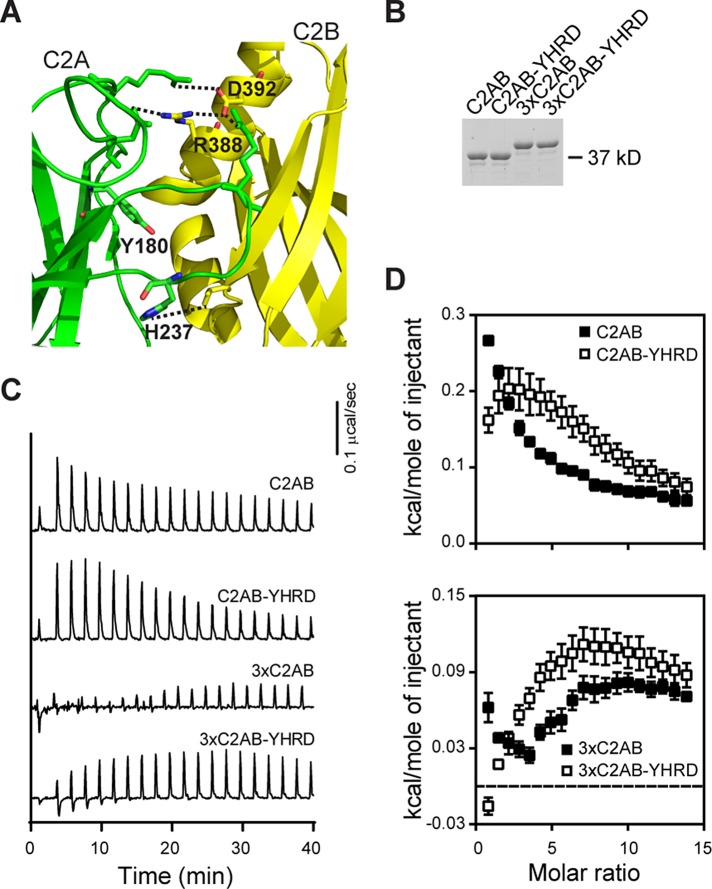
Mutations that disrupt putative inter–C2-domain interactions alter Ca2+ binding. (A) Structural model depicting four residues (Y180, H237, R388, and D392; interdomain interactions are shown as dotted lines) proposed to mediate interactions between the tandem C2 domains of syt-1 (C2A, green; C2B, yellow; from Figure 1A). These four residues, denoted YHRD, were mutated in a WT C2AB and a 3×C2AB mutant background. In the 3×C2AB construct, the native linker sequence between the C2A and C2B was tripled (Liu et al., 2014). (B) Representative SDS–PAGE gel of the proteins (0.6 μg) used in the ITC experiments. (C) Representative traces of the heat of Ca2+ binding to the indicated tandem C2 domain constructs; n ≥ 4. (D) Binding isotherms for WT C2AB (from Figure 1D), 3×C2AB, and YHRD mutant forms of each are shown for comparison; error bars represent SEM. Thermodynamic parameters are provided in Table 2.