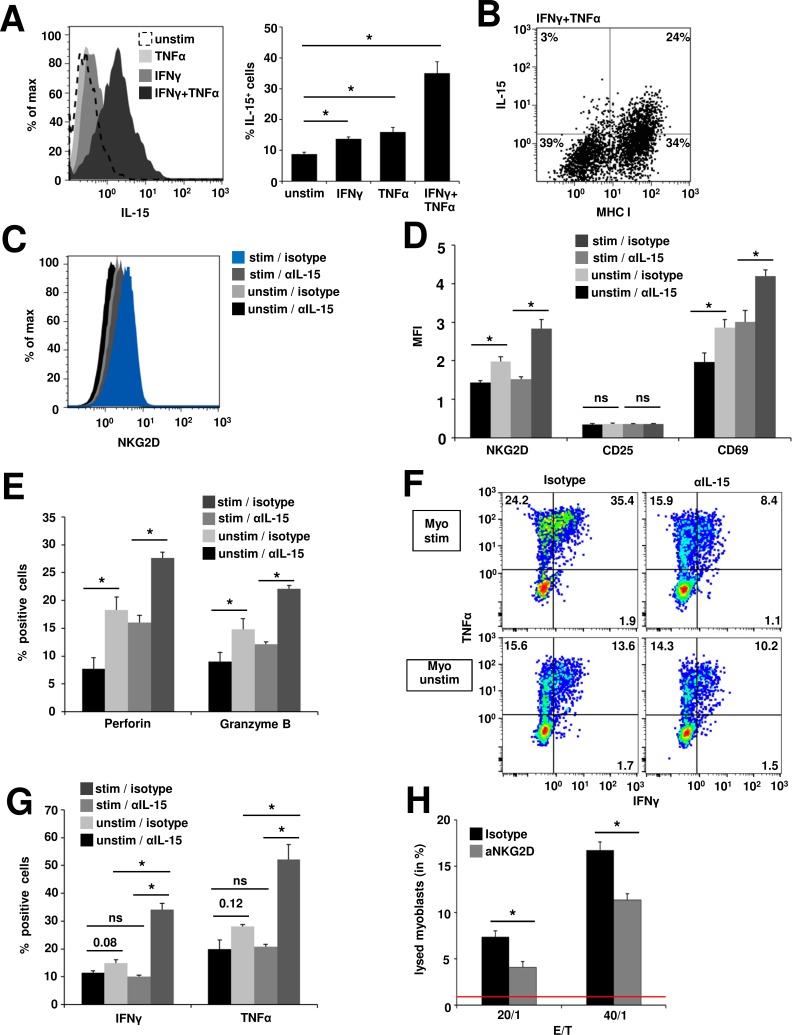
Figure 3. IL-15-dependent generation of cytotoxic CD8+NKG2Dhigh T cells in cocultures with inflamed human myoblasts.
A. Flow cytometry analysis of IL-15 surface expression on unstimulated and inflamed (IFNγ 1000 U/ml and/or TNFα 1000 U/ml, 48 h) human myoblasts. Left: Histogram depicting fluorescence intensity, right: percentage of IL-15+ myoblasts under different conditions. (n = 4) B. IL-15 expression positively correlates with MHC-I expression on IFNγ and TNFα treated myoblasts. A representative example is shown (n = 4) C. NKG2D expression on CD8+ T cells after 48 h coculture with unstimulated (unstim) or inflamed myoblasts (stim, IFNγ 1000 U/ml and TNFα 1000 U/ml, 48 h prior to coculture) in the presence of an IL-15 blocking antibody or isotype control (n = 4). IL-15-mediated upregulation of NKG2D is associated with increased T cell activation D., perforin and granzyme B expression (E. and pro-inflammatory cytokine production (F. + G.) (n = 4). H. 5 h FATAL assay of human inflamed (IFNγ: 1000 U/ml and TNFα: 1000 U/ml for 48 h prior to coculture), DiD (membrane dye) / CFSE (cytosolic dye) stained myoblasts and CD8+NKG2Dhigh T cells (pretreated and isolated from myoblast cocultures as described in C). Blocking anti-NKG2D antibody (10 μg/ml) or the respective isotype control (10 μg/ml) was added to the coculture 4 h prior and during the assay. Bar graphs depict the proportion of lysed myoblasts (DiD+CFSE−) (n = 4). Spontaneous lysis of myoblasts is indicated by the red line (0.87% ± 0.46%).* p < 0.05, ns = not significant, E:T = effector/target ratio.