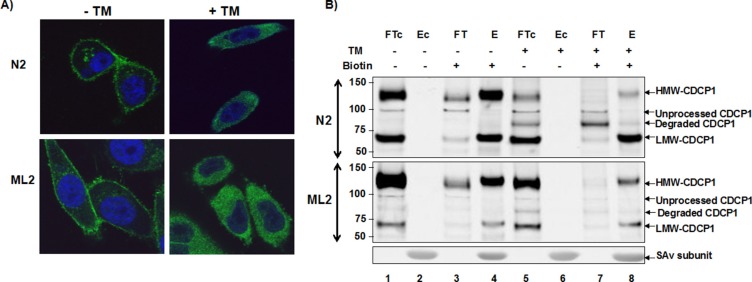
Figure 3. Surface expression of CDCP1 requires N-glycosylation.
(A) PC3-N2 and PC3-ML2 cells were either treated with 1 μg/ml tunicamycin for 24 h (+TM) or untreated (−TM). The cells were then fixed and subjected to immunofluorescence confocal microscopy using anti-CDCP1 antibody (CS4115, green). The nucleus was counterstained with TO-PRO3 (blue) and the images merged. (B) Protein extracts were prepared from PC3-N2 and ML2 cells that had been surface biotinylated (+) or untreated (−) and additionally grown in the presence (+) or absence (−) of tunicamycin. Biotinylated and nonbiotinylated protein fractions were separated by streptavidin affinity. The biotinylated bound (E) and unbound (FT) fractions, non-biotinylated bound (Ec) and unbound (FTc) fractions were separated by SDS-PAGE and immunoblotted with ant-CDCP1 (CS4115).