Abstract
Background
Fluorescent angiography (FA) has been useful for assessing blood flow and assessing tissue perfusion in ophthalmology and other surgical disciplines for decades. In plastic surgery, indocyanine green (ICG) dye-based FA is a relatively novel imaging technology with high potential in various applications. We review the various FA detector systems currently available and critically appraise its utility in breast reconstruction.
Methods
A review of the published English literature dating from 1950 to 2015 using databases, such as PubMed, Medline, Web of Science, and EMBASE was undertaken.
Results
In comparison to the old fluorescein dye, ICG has a superior side effect profile and can be accurately detected by various commercial devices, such as SPY Elite (Novadaq, Canada), FLARE (Curadel LLC, USA), PDE-Neo (Hamamatsu Photonics, Japan), Fluobeam 800 (Fluoptics, France), and IC-View (Pulsion Medical Systems AG, Germany). In breast reconstruction, ICG has established as a safer, more accurate tracer agent, in lieu of the traditional blue dyes, for detection of sentinel lymph nodes with radioactive isotopes (99m-Technetium). In prosthesis-based breast reconstruction, intraoperative assessment of the mastectomy skin flap to guide excision of hypoperfused areas translates to improved clinical outcomes. Similarly, in autologous breast reconstructions, FA can be utilized to detect poorly perfused areas of the free flap, evaluate microvascular anastomosis for patency, and assess SIEA vascular territory for use as an alternative free flap with minimal donor site morbidity.
Conclusions
ICG-based FA is a novel, useful tool for various applications in breast reconstruction. More studies with higher level of evidence are currently lacking to validate this technology.
Keywords: Fluorescent angiography (FA), indocyanine green (ICG), fluorescein, sentinel lymph node biopsy, mastectomy skin flap, perfusion, anastomotic patency, superficial inferior epigastric artery flap (SIEA flap)
Background
Breast cancer is the most common type of cancer worldwide and accounts for the most common cause of cancer-related deaths worldwide (1,2). More patients are electing to undergo mastectomy (3,4). As a result, postmastectomy breast reconstruction has become an essential component of the holistic management of patients with breast cancer. Encouraged by the advancement of imaging technologies in recent times, complex breast reconstructive procedures have become increasingly safer and more reliable (5-10).
Fluorescent angiography (FA) is a real-time imaging modality where an intravenous dye—namely, fluorescein and indocyanine green (ICG)—fluoresces and emits infrared energy upon excitation by a light source (11,12). It enables the assessment of blood flow and tissue perfusion in preoperative, intraoperative, and postoperative setting. For decades, FA has demonstrated useful in ophthalmic angiography (13-15), cardiothoracic surgery (16,17), hepatobilliary surgery (18,19), and neurosurgery (20).
Despite being readily accessible and affordable, fluorescein dye has a slow onset of action (15), prone to inter-observer variability (21,22), and has a significant side effect profile (23). As a result, it has been largely replaced by ICG. It has a superior side effect profile (22,24-28), shorter half-life (2.5 vs. 23.0 minutes), which enables multiple image captures (29), and is currently FDA-approved for evaluation of cardiac output, hepatic function, hepatic blood flow and ophthalmic vasculature (30). Currently, numerous commercial near-infrared (NIR) light detection devices are available (31). In breast reconstruction, SPY Elite System (Novadaq, Mississauga, Ontario, Canada), FLARE System (Curadel LLC, Worcester, MA, USA), PDE-Neo System (Hamamatsu Photonics, Hamamatsu, Japan), Fluobeam 800 System (Fluoptics, Grenoble, France), and IC-View System (Pulsion Medical Systems AG, Munich, Germany) have been studied, but SPY Elite System has been most frequently reported sensor (32-35).
In plastic surgery, FA has been used for various indications (35-43). However, its application has been most extensively reported in breast reconstructive surgery and, in this review, we investigate the role of FA in sentinel lymph node biopsy, prosthesis-based reconstructions, and autologous free flap reconstructions.
Methods
We reviewed the published English literature from 1950 to 2015 from well-established databases, such as PubMed, Medline, Web of Science, and EMBASE, using the following search terms in various combinations: “fluorescent angiography”, “indocyanine green”, “fluorescein”, “sentinel lymph node biopsy”, “mastectomy skin flap”, “prosthesis breast reconstruction”, “autologous breast reconstruction”, “DIEP”, “TRAM”, “flap perfusion”, “anastomotic patency”, and “SIEA”.
Results
Fluorescent angiography (FA)
FA is a real-time imaging modality where an intravenous dye fluoresces and emits infrared energy upon excitation by a light source (11,12). As a result, it enables the assessment of blood flow and tissue perfusion in preoperative, intraoperative, and postoperative setting. To date, investigators have demonstrated its utility in various surgical disciplines, such as ophthalmology to evaluate the chorioretinal vasculature (13-15), cardiothoracic surgery to assess the patency of the coronary artery bypass graft (16,17), hepatobilliary surgery to identify the haptic segment and subsegment for anatomical hepatic resection (18,19), and neurosurgery to assess the patency of the superficial temporal artery-middle cerebral artery bypass graft in cerebral revascularization procedure (20). Historically, two fluorescent dyes have been studied for FA: fluorescein and ICG (Table 1), along with numerous commercial detector devices (Table 2).
Table 1. Comparison of fluorescein and ICG dye (11,15).
| Characteristic | Fluroescein | ICG |
|---|---|---|
| Molecular weight | 375 kD | 775 kD |
| Absorption wavelength | 494 nm | 800 nm |
| Emission wavelength | 521 nm | 830 nm |
| Half-life | 23 min | 2.5 min |
| Protein-binding | + | +++ |
| Excretion | Urinary | Biliary |
| Waiting time after injection | 15 min | 2 min |
| Data | Qualitative | Quantitative |
| Side effect profile | ++ | + |
ICG, indocyanine green; kD, kilodalton; nm, nanometer.
Table 2. Comparison of fluorescent angiography devices used in breast reconstruction.
| Feature | SPY Elite | FLARE | PDE-Neo | Fluobeam 800 | IC-View |
|---|---|---|---|---|---|
| Cost per case (USD) | 650 | 109 | 60 | 13 | N/A |
| Cost of machine (USD) | 250,000 | 120,000 | 75,000 | 40,000 | N/A |
| Structure | Trolley with articulating arm | Trolley with articulating arm | Handheld | Suspension equipment | Handheld |
| Working distance (cm) | 34 | 45 | 25 | 20 | 100 |
| Field view (cm2) | 10.1×7.4 | 15×11 | 13×9 | 10×7.5 | 40×20 |
| Pixel | 300,000 | 307,200 | 300,000 | 316,000 | N/A |
| Resolution | 30 fps | 30 fps | 2.83 cycles/mm | 4 cycles/mm | N/A |
| Zoom | No | Yes | No | No | N/A |
| Operating room light compatibility | No | Yes | No | Yes | N/A |
| Data | Quantitative | Quantitative | Qualitative | Qualitative | Quantitative |
| Software | SPY-Q | XIP | None | None | IC-CALC |
FLARE, Fluorescence-Assisted Resection and Exploration; PDE, photodynamic element; N/A, not available; fps, frames per second; XIP, eXtensible Imaging Platform.
Dyes
Fluorescein
Early studies have used fluorescein dye, which is readily accessible and affordable. Upon exposure to UV light using the Woods lamp, it emits yellow-green fluorescent light and is really excreted. It was first synthesized and used topically by von Baeyer to diagnose corneal abrasions (44). Since then, it has been utilized widely to assess skin flap perfusion and viability (23,28,45-48). One of the major disadvantages of fluorescein lies in its slow action, requiring 15 minutes to reach maximum fluorescence, and potentially increasing operating time, exposure to general anaesthesia, and operating cost (15). Furthermore, fluorescein yields qualitative data, which is subject to inter-observer variability (21,22). In addition, it has a significant side effect profile, a high rate of allergic reaction (0.6%) (23), and the use of the Woods lamp has a steep learning curve. As a result, fluorescein has been largely replaced by ICG dyes.
Indocyanine green (ICG)
ICG is a water-soluble, biliary-excreted dye that is excited by laser light and emits infrared energy within 2 minutes of intravenous injection (11). Kogure et al. first used it to image choroidal veins (49) and, currently, it is approved by the US Food and Drug Administration (FDA) to evaluate cardiac output, hepatic function, hepatic blood flow and ophthalmic vasculature (30). In comparison to fluorescein, ICG is a larger molecule (775 vs. 375 kD) and binds to plasma proteins more strongly (50). As a result, it stays in the intravascular compartment and undergoes rapid washout from the circulation leading to a superior side effect profile (22,24-28). Furthermore, ICG has a shorter half-life than fluorescein (2.5 vs. 23 minutes), which enables multiple image captures intraoperatively (29). However, one of the major limiting factors of ICG is that with the current technology the emitted light can only be detected up to 1 cm deep (31).
Devices
Numerous commercial NIR light detection devices can be used to derive quantitative data from the ICG-derived fluorescence (31). In breast reconstruction, SPY Elite System (Novadaq), FLARE System (Curadel LLC), PDE-Neo System (Hamamatsu Photonics), Fluobeam 800 System (Fluoptics), and IC-View System (Pulsion Medical Systems AG) have been studied (Table 2).
SPY Elite system
SPY Elite system is the most extensively reported NIR light detection device in breast reconstructive surgery (32-35). The machine is mounted on to a trolley and the illumination probe is attached to an articulating arm. Compared to the other commercial devices, SPY System has a smaller field of view (10.1×7.4 cm2) and lacks operating room light compatibility, which makes it cumbersome for a regular use. The data is analyzed quantitatively using the proprietary SPY-Q software (Novadaq, Mississauga, Ontario, Canada). It allows the storage of both static pictures and videos, and moreover, clinicians can perform various quantitative analyses on it. However, it incurs a significant learning curve. To date, SPY Elite System is FDA-approved for applications in plastic and reconstructive surgery, cardiothoracic surgery, visceral surgery, and transplant surgery (51).
FLARE system
In contrast to most of the FA devices, Fluorescence-Assisted Resection and Exploration (FLARETM) system consists of two NIR cameras that are capable of detecting multiple wavelengths, both ICG and methylene blue, and are synchronized to a color video camera (52). The NIR and color images are overlaid using eXtensible Imaging Platform (XIP) (Electronic Radiology Lab, St Louis, MO, USA), an open-source software developed by the National Cancer Institute (NCI) (53). The merged images display real-time tissue fluorescence in the context of the surgical anatomy (54). Furthermore, FLARE has the longest working distance (45 cm) and the largest field view (15×11 cm2), compared to all the other systems (Table 2). To date, FLARE has been reported useful in detecting sentinel lymph nodes (54) and assessing free flap perfusion and perforator selection in animal models (39).
PDE-Neo system
Photodynamic Eye (PDE) system is the oldest FA device available in the market and has been recently upgraded to the PDE-Neo system. It is manufactured in a handheld probe that contains a series of light-emitting diodes (LED) and a charge-coupled device (CCD) sensor that filters out wavelengths of less than 820 nm. PDE-Neo is relatively affordable and has an adequate working distance (25 cm) and a field of view (13×9 cm2). However, it is restricted to only producing qualitative data and is not compatible with operating theater lights. Numerous investigators have reported the utility of PDE-Neo in sentinel lymph node dissection, evaluation of microvascular anastomosis, and skin flap perfusion (38,55-59).
Fluobeam 800 system
Fluobeam 800 system is a novel and affordable NIR detector developed in France. The device is small and can be fitted into a suspension equipment found in an operating room. Moreover, it is compatible with the operating room light. However, in comparison to the other systems, it has the shortest working distance (20 cm) and a small field of view (10×7.5 cm2). Furthermore, it only produces subjective qualitative information. Currently, only preclinical studies in animal models of skin flaps have been reported using this device (60-63).
IC-View system
Similar to the PDE-Neo system, IC View is manufactured in a handheld probe. Amongst all commercial FA devices, it has the farthest working distance (100 cm) and the largest field of view (40×20 cm2). Furthermore, its accompanying proprietary IC-CALC software (Pulsion Medical Systems AG, Munich, Germany) enables quantitative analysis. Investigators have utilized this system to demonstrate mesenteric vasculature (64), bowel wall vasculature (65), perfusion of kidney allograft (66) and free flaps (42,67-69), sentinel lymph node diagnosis (31,70-72), and deep inferior epigastric artery perforator (DIEP) flap monitoring (73). However, it is no longer available in the market.
FA in breast reconstruction
In plastic and reconstructive surgery, FA has so far demonstrated utility in the assessment of breast reduction skin flaps (36,37), selection of dominant perforator in free flap transfers (35,38), assessment of flap perfusion (39,40) and patency of microvascular anastomosis (41), postoperative flap monitoring (42), and in the assessment of burns injury (43). To date, its application has been most extensively explored and reported in breast reconstructive surgery, mainly to aid sentinel lymph node biopsy, evaluate mastectomy skin flaps in prosthesis-based reconstructions, and autologous free flap reconstructions.
Sentinel lymph node biopsy
Sentinel lymph node biopsy is a crucial component of axillary staging in patients with breast cancer (74,75). Accurate diagnosis is paramount since axillary clearance in sentinel node-negative patients has demonstrated no clinical benefits but creates morbidity from the subsequent lymphedema (74). Currently, the gold standard investigation involves using methylene or patent blue dye for node detection and radioisotope for lymphoscintigraphy (74,76,77). However, the blue dye is associated with cutaneous staining and a significant rate of allergic reaction and anaphylaxis (75). In contrast, ICG is a safe, accurate, and reliable tracer agent (57,70,71,78,79) (Table 3).
Table 3. Summary of all clinical studies comparing the node detection rate (i.e., over the total number of patients) and node uptake rate (i.e., over the total number of sentinel lymph nodes detected).
| Author | Year | P/R | Patient | Nodes | Node detection rate (%) |
Node uptake rate (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blue | ICG | 99mTc | Blue + ICG | ICG + 99mTc | Blue | ICG | 99mTc | Blue + ICG | Blue + 99mTc | ICG + 99mTc | ||||||
| Sugie (80) | 2015 | P | 821 | N/A | N/A | 97.2 | 97 | N/A | 99.8 | N/A | 93.3 | 90 | N/A | N/A | N/A | |
| Samorani (81) | 2015 | P | 301 | 589 | N/A | 98.7 | 95.4 | N/A | 98.7 | N/A | 99 | 77.7 | N/A | N/A | 76.7 | |
| Pitsinis (56) | 2015 | P | 50 | 87 | 96 | 96 | N/A | 96 | N/A | 96.6 | 100 | N/A | 96.6 | N/A | N/A | |
| Verbeek (82) | 2014 | P | 95 | 177 | N/A | 99 | N/A | N/A | 99 | 78 | 100 | 88 | N/A | N/A | N/A | |
| Ballardini (55) | 2013 | P | 134 | 246 | N/A | N/A | N/A | N/A | N/A | N/A | 99.6 | 93.9 | N/A | N/A | N/A | |
| Wishant (59) | 2012 | P | 99 | 242 | 99 | 100 | 91.3 | N/A | N/A | 78.9 | 83.1 | 64.5 | 95 | 73.1 | N/A | |
| Tagaya (57) | 2011 | P | 50 | N/A | N/A | 100 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Sugie (71) | 2010 | P | 411 | 950 | 93 | 99 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Murawa (70) | 2009 | P | 30 | N/A | N/A | 96.7 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Kitai (78) | 2005 | P | 18 | 50 | N/A | 94 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
P, prospective; R, retrospective; ICG, indocyanine green; 99mTc, technetium radioisotope; N/A, not available.
ICG facilitates a superior means of sentinel lymph node dissection from three-dimensional (3D) visualization of the lymphatic vasculature, as shown in animal models (54). Furthermore, investigators have reported that ICG may be more accurate than methylene blue (83) and its nodal detection rate may be decreased if its use is combined with the blue dye (56,59). Compared to the radioisotope, ICG has a similar nodal detection rate (55,80,83). In combination with radioisotopes, ICG have shown to improve the sensitivity of detecting positive sentinel nodes (80,82). Recently, Samorani et al. have demonstrated, in a prospective study involving 301 patients and 589 lymph nodes excised, that ICG has a superior node detection rate than technetium, a standard radioisotope (99% vs. 76.7%) (81). Interestingly, Onishi et al. report that the nodal detection rate of ICG can be enhanced by incubating it with human serum albumin (HSA) to form a 1:1 complex, which increases its quantum yield (i.e., fluorescence signal) and its hydrodynamic diameter (i.e., nodal retention) (54,84). However, this novel development currently requires more investigation. In summary, ICG has become more established as an alternative tracer agent to the traditional blue dye for investigation with radioactive isotope in sentinel lymph node biopsy.
Prosthesis-based breast reconstruction
In the recent decades, the rate of prosthesis-based breast reconstruction has increased rapidly (203% rise from 1998 to 2008) and has exceeded autologous reconstruction as the most common approach, accounting for 65% of all breast reconstructions (85). To date, two-stage tissue expander approach has been the most common mode of implant reconstructions (86). However, with the advancement of surgical techniques and adjuvant technologies, single-stage implant reconstruction is becoming more popular where indicated (87-89). One-stage procedure simplifies the reconstructive process, limits exposure to general anesthesia and is potentially more cost-effective (90,91). Yet, majority of clinicians still perform the two-stage reconstruction due to a relatively high risk of implant-related complications and prosthesis failure associated with the single-stage procedure (92-94).
Overall, postmastectomy implant reconstructions are associated with 40–50% of complications and the most common complication is mastectomy skin flap necrosis (32,95-98). The risk is higher in smokers, obese patients, large breasts, and adjuvant radiotherapy (93). Subsequently, this can lead to an implant failure or loss (99). Similarly, timely re-exploration and necrosis excision can salvage the reconstruction (32). Some clinicians have utilized acellular dermal matrix to reduce the skin tension and prevent implant exposure, but with minimal improvement in the overall rate of mastectomy skin flap necrosis and (6.9%) and implant loss (1.1%) (98). To this effect, using FA to facilitate intraoperative assessment of the mastectomy skin perfusion and guide appropriate excision has been studied to be beneficial (100) (Table 4).
Table 4. Summary of all clinical studies reporting the utility of ICG in detecting mastectomy skin flap necrosis in prosthesis-based breast reconstructions.
| Author | Year | Implants | Patient | Control | P/R | Device | Rate of implant-related necrosis (%) | Findings |
|---|---|---|---|---|---|---|---|---|
| Kanuri (99) | 2014 | 710 | 508 | N/A | R | SPY | 11.1 | Indiscriminate used of ICG is more expensive than the complication-related costs by 65% |
| Cost savings per flap necrosis prevented: USD 2,098.80 for smokers, USD 5,162.30 for BMI >30, USD 1,892.70 for mastectomy weight >800 g | ||||||||
| Duggal (94) | 2014 | N/A | 184 | 184 | R | SPY | 13 | ICG decreased necrosis (13% vs. 23.4%, P=0.01) |
| Cost savings per patient: USD 614 | ||||||||
| Munabi (101) | 2014 | 50 | 42 | N/ | P | SPY | 12 | SPY-Q perfusion unit ≤7.0: 88% sensitivity, 83% specificity |
| SPY-Q perfusion unit ≤10.0: 100% sensitivity, 72% specificity | ||||||||
| 18% false positive rate | ||||||||
| Phillips (93) | 2012 | 51 | 32 | N/A | P | SPY | 41.2 | ICG: 90% sensitivity, 50% specificity for necrosis |
| ICG overpredicts hypoperfusion by 66.57 cm2 | ||||||||
| SPY-Q perfusion unit <3.7: 90% sensitivity, 100% specificity for necrosis | ||||||||
| SPY-Q perfusion unit <8.0: 100% sensitivity, 70% specificity for necrosis | ||||||||
| Moyer (100) | 2012 | 15 | 14 | N/A | R | SPY | 14 | SPY-Q perfusion score ≤25%: 90% sensitivity for necrosis |
| SPY-Q perfusion score ≥45%: 98% sensitivity for survival | ||||||||
| SPY-Q perfusion score 33%: 88% PPV for necrosis, 16% NPV for survival | ||||||||
| Newman (102) | 2010 | 19 | 11 | N/A | R | SPY | 45 | 95% correlation between ICG and postoperative necrosis |
| 100% sensitivity, 91% specificity | ||||||||
| Komorowska-Timek (33) | 2010 | 16 | 12 | 148 | P | SPY | 4 | ICG decreased necrosis (4% vs. 15.1%, P<0.01) |
ICG, indocyanine green; P, prospective; R, retrospective; N/A, not available; BMI, body mass index; PPV, positive predictive value; NPV, negative predictive value.
Fluorescein
In a case series, Singer et al. first reported the use of fluorescein-based FA as an adjunct to assess the viability of the mastectomy skin flap in implant reconstructions (103). However, the authors noted that the extent of necrosis could not be accurately predicted using fluorescein and the hypofluorescent areas underestimated the tissue survival. Using fiberoptic dermofluorometer on various animal models of skin flaps, investigators were able to quantitate fluorescence and produce numerous formulae to predict tissue necrosis and survival, such as perfusion ratio (22), dye fluorescence index (DFI) (48,104), and fluorescein flowmetry (105). The manual nature of the operation of the sensors meant that the result is prone to subjective interpretation and selection bias (22). Interestingly, Losken et al. attempted to classify the fluorescence pattern categorically into homogenous yellow, mottled appearance, and complete failure of fluorescence (21). The authors demonstrated that their algorithm was highly predictive of flap survival, but would overestimate flap necrosis (21). With the introduction of ICG, Phillips et al. compared the two dyes in a prospective study involving 51 implant reconstructions in 32 patients (93). They showed that both fluorescein and ICG have similar sensitivity (90%) in detecting skin flap necrosis, but ICG has a superior specificity (P=0.002) (93).
ICG
An earlier study by De Lorenzi et al. demonstrated that ICG-based FA is a safe, accurate and quick method of determining soft tissue perfusion intraoperatively in implant breast reconstructions (106). In a retrospective study of 19 prosthesis-based reconstructions using acellular dermal matrix, Newman et al. reported the potential benefits of ICG in identifying subclinical mastectomy skin flap necrosis (102). From their retrospective analysis of intraoperative FA images, the authors noted a 95% correlation between ICG findings and subsequent skin necrosis with sensitivity and specificity of 100% and 91%, respectively (102).
Subsequently, investigators have attempted to define threshold values, using the SPY-Q software, which enables reliable prediction of mastectomy skin flap perfusion and necrosis (93,100,101). Moyer et al. utilized the SPY-Q perfusion score where the fluorescence is recorded relative to the surrounding well-perfused tissues designated as “100% fluorescent” (100). They showed that a score of less than 33% has a positive predictive value of 88% for necrosis and a negative predictive value of 16% for survival (100). However, this scoring system was prone to inter-observer variability. In contrast, Phillips et al. and Munabi et al. employed absolute measurement of tissue fluorescence to derive sensitivity and specificity of ICG (93,101). However, the authors also documented that this measurement can be confounded in smokers and in the presence of epinephrine containing tumescent solution (101). As yet, a standardized, reliable and reproducible threshold value has not been established.
Current evidences indicate that using ICG-based FA to guide excision of hypoperfused mastectomy skin flap in the operating room results in a significant reduction in necrosis (33,94). Furthermore, in comparison to the historical control, Duggal et al. report a reduction in the severity of the skin flap necrosis (25% vs. 44.1%) and also the rate of re-operation (6% vs. 14%) (94). These findings translate to a cost saving of USD 614 per patient (94). However, given the high cost of leasing or purchasing an ICG detector device currently, Kanuri et al. show that an indiscriminate use of ICG in all breast reconstructions is more expensive than cost related to flap complications by 65% (99). They recommend that reserving the use of FA in high-risk patients—smokers, body mass index (BMI) of greater than 30 kg/m2, and mastectomy weight of greater than 800 g—leads to significant cost-savings (99).
Autologous breast reconstruction
In contrast to the prosthesis-based reconstructions, autologous breast reconstructions using perforator-based free flaps are in general more natural-appearing, aesthetically pleasing, and long-lasting (107,108). Historically, various donor sites have been studied for breast volume replacement, such as omentum (109), gluteal artery perforator flap (110), latissimus dorsi (111), deep circumflex iliac artery (groin) flaps (112), tensor fascia latae (lateral thigh) flap (113), triceps flap (114), superficial inferior epigastric artery (SIEA) perforator flap (115), transverse upper gracilis (TUG) flap (116), and profunda femoris artery perforator (PAP) flap (117). However, the abdominal wall remains the most popular donor site due to its consistent large volume and the added aesthetic benefit to the donor site, akin to an abdominoplasty. Originally, transverse rectus abdominis muscle (TRAM) flaps have been successful at providing sufficient volume in breast reconstructions (118-120). However, they are associated with a significant number of partial flap necrosis (7%–31%), especially in the periphery of the flap, and donor site morbidity from subsequent rectus muscle weakness and ventral hernia (0.3%–11.0%) (121,122). As a result, muscle-sparing techniques have evolved and DIEP flaps have become popular.
The advent of preoperative planning with advanced modern imaging modalities has assisted surgeons in the appropriate selection of donor site, perforator, and flap, and led to improved flap outcomes (9,123). To this effect, ICG-based FA has been studied to evaluate perforator caliber and location for breast reconstructions (38,124). However, since FA can only assess tissues up to 1 cm deep and thick pannus is preferred in DIEP flaps, it is less frequently utilized in the preoperative setting, compared to computed tomographic angiography (CTA) and magnetic resonance angiography (MRA) (8-10,125-129). Similarly, FA has been explored as a postoperative free flap monitoring tool in the postoperative setting (48). Despite promising early results (12,130), Mothes et al. demonstrate that ICG-based FA is not superior to the conventional clinical monitoring techniques (42). Hitherto, in autologous breast reconstruction, FA has been most beneficial in the intraoperative setting for the assessment of free flap perfusion and blood flow across microvascular anastomosis in a range of free flaps.
Flap perfusion
Numerous studies have reported that ICG-indicated hypoperfused areas correlate accurately with areas of postoperative flap necrosis in TRAM and DIEP flaps (33-35,131-133). Francisco et al. describe, in a case series of five DIEP flaps, their technique of using ICG during flap insetting to determine poorly perfused areas and guide excision (124). Newman et al. note that flap adjustments performed in three out of four cases, where FA demonstrated hypoperfusion, led to successful reconstruction and no postoperative complications (34). Recently, Duggal et al. have retrospectively compared the clinical outcomes from ICG-guided excision in 71 consecutive TRAM flaps to 59 flaps performed prior to the introduction of FA in a single institution (94). The authors report a trend in the reduction of the rate of flap necrosis (14% vs. 22%, P=0.237) and flap loss (1.4% vs. 3.4%, P=1.00). Although promising, more studies with higher power and a stronger level of evidence are required to validate the use of ICG for intraoperative assessment of free flap perfusion in breast reconstruction (Table 5) (Figure 1).
Table 5. Summary of all clinical studies reporting the utility of ICG in detecting flap and fat necrosis in autologous breast reconstructions.
| Author | Year | Type | Auto | Patient | Control | P/R | Device | Rate of necrosis (%) | Findings |
|---|---|---|---|---|---|---|---|---|---|
| Duggal (94) | 2014 | TRAM | 71 | N/A | 59 | R | SPY | 10 | ICG-guided excision showed a trend in the reduction of TRAM flap necrosis (14% vs. 22%, P=0.237) and flap loss (1.4% vs. 3.4%, P=1.00) |
| Wu (131) | 2013 | TRAM, DIEP | N/A | 17 | N/A | R | SPY | 7 | Overestimation of perfusion 7% |
| Underestimation of perfusion 7% | |||||||||
| ICG may be more unreliable in patients with dark skin (overestimation of 22% and underestimation of 22%) | |||||||||
| Komorowska-Timek (33) | 2010 | TRAM, DIEP, SIEA, LD | 8 | 8 | 148 | P | SPY | 4 | 1 case of partial necrosis occurred reconstruction with LD flap and tissue expander where ICG detected subclinical hypoperfusion |
| Francisco (124) | 2010 | DIEP | 5 | N/A | N/A | R | SPY | 0 | ICG is useful for perforator selection, SIEA assessment, microvascular anastomosis patency assessment, and flap perfusion assessment |
| Jones (132) | 2009 | TRAM, DIEP, LD | 64 | 43 | N/A | R | SPY | 6.3 | ICG correlated 100% with areas of necrosis |
| Newman (34) | 2009 | TRAM,DIEP | 10 | 8 | N/A | P | SPY | N/A | 3 out of 4 flaps showing poor perfusion by ICG were improved by intraoperative adjustments |
| 1 out of 4 flaps showing poor perfusion by ICG was not adjusted intraoperatively and required re-exploration | |||||||||
| Pestana (35) | 2009 | TRAM, DIEP, SGAP, SIEA, TUG | 12 | 10 | N/A | R | SPY | 8 | ICG is safe and requires minimal additional operative time and general anesthesia |
| Yamaguchi (133) | 2004 | TRAM | 10 | 10 | N/A | R | IC-View | 40 | ICG accurately predicted 3 out 4 cases of partial necrosis |
ICG, indocyanine green; auto, number of autologous breast reconstructions; P, prospective; R, retrospective; TRAM, transverse rectus abdominis muscle; LD, latissimus dorsi; DIEP, deep inferior epigastric artery perforator; SIEA, superficial inferior epigastric artery; SGAP, superior gluteal artery perforator; TUG, transverse upper gracilis.
Figure 1.
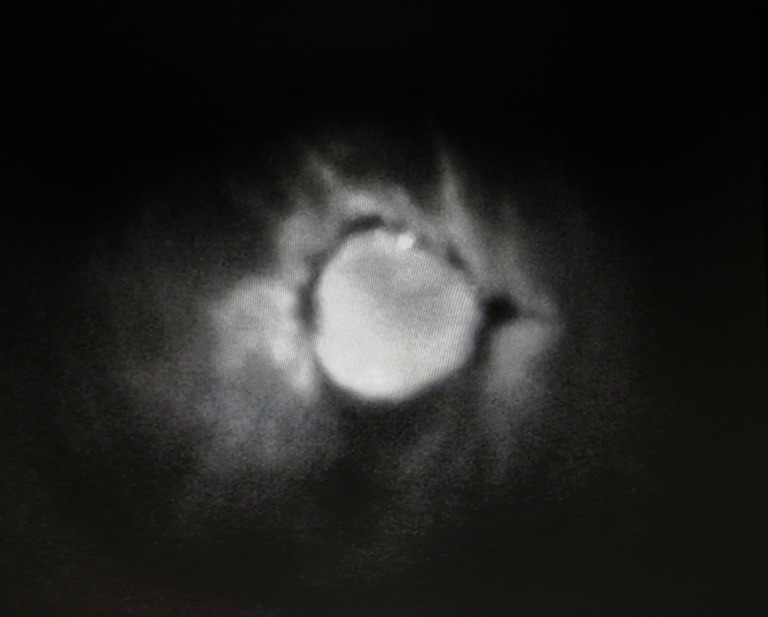
Indocyanine green (ICG) immunofluorescence intraoperative screen of a deep inferior epigastric artery perforator (DIEP) flap and surrounding mastectomy flaps. The flap is seen well-perfused, while an infra-flap area of mastectomy skin is poorly perfused.
Microvascular anastomotic patency
FA enables direct visualization of blood flow through microvascular anastomosis and facilitates assessment of its patency. Flow defect may indicate arterial occlusion, venous thrombosis, or pedicle torsion (134). In a retrospective analysis of 50 consecutive free flap reconstructions, including DIEP flaps, 22% of anastomosis at re-exploration appeared occluded using ICG-based FA but clinically patent (135). Where clinical reasoning suggested temporary causes, such as vasospasm and reversible venous thrombosis, accounted for the subclinical vascular compromise, the anastomosis was left unrevised. All three cases resulted in flap loss or re-exploration. Later study by Holm et al. demonstrated that FA can detect thrombotic occlusions with sensitivity of 100% and specificity of 86% (136). Subsequently, the same group calculated the anastomotic intrinsic transit time (ITT), the time taken for the dye to travel from the arterial anastomosis to the venous anastomosis, and applied it to 100 consecutive free flaps including autologous breast reconstructions to predict vascular compromise (137). ITT of 50 seconds or greater would indicate postoperative re-exploration or flap loss with sensitivity of 92% and specificity of 98%.
SIEA flaps
Despite having no donor site morbidity, SIEA flap has been less commonly utilized due to its inconsistent vascular anatomy and variable size of its vascular territory (138,139). However, more recent cadaveric and clinical studies suggest that SIEA may be present more consistently and approximately in 27–35% of all patients (115,140-144). Numerous reports devised guidelines that SIEA flaps are only indicated for breast reconstruction when its diameter is greater than 1 or 1.5 mm at the origin (115,141,144,145). However, these guidelines are oversimplified and fail to acknowledge individual variances. Ulusal et al. and Holm et al. used laser Doppler flowmetry and ICG-based FA, respectively, to determine the vascular territory of SIEA and design its flap (142,143). In the following study, Holm et al. proposed a management algorithm where SIEA flap is indicated when sufficient vessel caliber and vascular territory are present using intraoperative FA (146). Where SIEA is not indicated, the algorithm recommended translation to a DIEP flap or a stacked flap involving both the deep and the superficial system. However, more evidences are still required to validate this algorithm.
Discussion
Breast cancer is the most common type of cancer worldwide and accounts for the most common cause of cancer-related deaths worldwide (1,2). Approximately 25% of the patients will undergo mastectomy (3) and an increasing number of patients are opting for more aggressive types of mastectomy (4). As a result, postmastectomy breast reconstruction has become an essential component of the holistic care in patients with breast cancer. Encouraged by the advancement of imaging technologies, complex breast reconstructive procedures have become increasingly safe and reliable (5-10). FA is such imaging modality that has been studied to show usefulness in breast reconstructions.
FA is a real-time imaging modality where an intravenous dye—mainly, fluorescein and ICG—fluoresces and emits infrared energy upon excitation by a light source (11,12). It enables the assessment of blood flow and tissue perfusion in preoperative, intraoperative, and postoperative setting. To date, investigators have demonstrated its utility in ophthalmic angiography (13-15), cardiothoracic surgery (16,17), hepatobilliary surgery (18,19), and neurosurgery (20). Despite being readily accessible and affordable, fluorescein has a slow onset of action, requiring 15 minutes to reach maximum fluorescence (15). This potentially increases the operating time, exposure to general anaesthesia, and consequently the operating cost (Table 1). Furthermore, fluorescein produces only qualitative data, subject to inter-observer variability (21,22), and has a significant side effect profile (23). As a result, it has been largely replaced by ICG dyes. ICG is a water-soluble, biliary-excreted dye that is already FDA-approved to evaluate cardiac output, hepatic function, hepatic blood flow and ophthalmic vasculature (30). In comparison to fluorescein, ICG has a superior side effect profile (22,24-28) and a shorter half-life than fluorescein (2.5 vs. 23.0 minutes), which enables multiple image captures (29). One of the major drawbacks of ICG is that the emitted light can only be detected up to 1 cm deep (31).
Numerous commercial NIR light detection devices are currently available (31). In breast reconstruction, SPY Elite system (Novadaq), FLARE system (Curadel LLC), PDE-Neo system (Hamamatsu Photonics), Fluobeam 800 system (Fluoptics), and IC-View system (Pulsion Medical systems AG) have been studied (Table 2). SPY Elite system has been most frequently reported (32-35). Despite having a relatively small field of view (10.1×7.4 cm2) and lacking operating room light compatibility, in conjunction with the accompanying SPY-Q software (Novadaq), it allows storage of both static pictures and videos and performs complex quantitative analyses. To date, SPY Elite System has been FDA-approved for applications in plastic and reconstructive surgery, cardiothoracic surgery, visceral surgery, and transplant surgery (51). In contrast to the other devices, FLARE system consists of two separate NIR cameras synchronized to a color video camera, which enables display of real-time tissue fluorescence in the context of the surgical anatomy (54). Furthermore, FLARE has the longest working distance (45 cm) and the largest field view (15×11 cm2). To date, the evaluation of FLARE system has been limited to preclinical animal models (39,54). PDE system has been available in the market longest. It is relatively affordable and has respectable specifications. However, it only yields qualitative data and is not compatible with operating theater lights (38,55-59). Fluobeam 800 system is a small novel device that can be fitted into a suspension equipment in an operating theater. However, it produces qualitative data and has the shortest working distance (20 cm) and the smallest field of view (10×7.5 cm2). Furthermore, similar to the FLARE system, it has only been investigated in animal models (60-63). IC-View has been well studied (31,42,64-73), but unfortunately, it is no longer available.
In plastic surgery, FA has been useful in the assessment of breast reduction skin flaps (36,37), selection of dominant perforator in free flap transfers (35,38), assessment of flap perfusion (39,40) and patency of microvascular anastomosis (41), postoperative flap monitoring (42), and in the assessment of burns injury (43). However, its application has been most extensively reported in breast reconstructive surgery, to aid sentinel lymph node biopsy, evaluate mastectomy skin flaps in prosthesis-based reconstructions, and autologous free flap reconstructions.
Sentinel lymph node biopsy is a crucial component of axillary staging in patients with breast cancer (74,75). Accurate diagnosis is paramount since axillary clearance in sentinel node-negative patients has demonstrated no clinical benefits but creates morbidity from the subsequent lymphedema (74). Currently, the gold standard investigation involves the use of methylene or patent blue dye for node detection and radioisotope for lymphoscintigraphy (74,76,77). However, the blue dye has a high side effect profile (75). In contrast, ICG is a safe, accurate, and reliable tracer agent (56,57,59,70,71,79-83) (Table 3). Furthermore, ICG enables 3D visualization of the lymphatic vasculature (54). Compared to the radioisotope, ICG has a similar or superior nodal detection rate (55,80,81,83) and when they are combined, the rate of positive sentinel node detection is improved (80,82). Moreover, the nodal detection rate of ICG can be enhanced by incubating it with HSA (54,84). In summary, ICG is a superior tracer agent to the traditional blue dye for investigation with radioactive isotope in sentinel lymph node biopsy.
The rate of prosthesis-based breast reconstruction is rising rapidly (85). In comparison to the traditional two-stage tissue expander approach, a single-stage implant reconstruction is potentially more attractive since it simplifies the reconstructive process, limits exposure to general anesthesia and hence, more cost-effective (87-91). However, majority of clinicians still perform the two-stage reconstruction due to relatively high risk of implant-related complications and prosthesis failure associated with the single-stage procedure (92-94). Using acellular dermal matrix has only demonstrated small benefit in the overall rate of mastectomy skin flap necrosis and (6.9%) and implant loss (1.1%) (98). To this effect, FA can useful by enabling clinicians to detect subclinical hypoperfused tissue and guide appropriate excision (100,102,106) (Table 4). Numerous studies have attempted to define a threshold value using both relative and absolute measurements to reliably predict perfusion and potential necrosis (93,100,101). However, a standardized, reproducible threshold value has not been established. Nonetheless, evidences indicate that using ICG-based FA to guide excision of hypoperfused mastectomy skin flap results in a significant reduction in skin necrosis (33,94). Furthermore, Duggal et al. report a reduction in the severity of skin flap necrosis (25% vs. 44.1%) and the rate of re-operation (6% vs. 14%), compared to the historical control (94). Additionally, these findings translate to a cost saving of USD 614 per patient (94). However, Kanuri et al. mention that an indiscriminate use of ICG can be expensive and recommend reserving the use of FA in high-risk patients—smokers, BMI of greater than 30 kg/m2, and mastectomy weight of greater than 800 g (99).
In contrast to the prosthesis-based reconstructions, autologous breast reconstructions are more natural-appearing, aesthetically pleasing, and long-lasting (107,108). Historically, various donor sites have been studied for breast volume replacement, such as omentum (109), gluteal artery perforator flap (110), latissimus dorsi (111), deep circumflex iliac artery (groin) flaps (112), tensor fascia latae (lateral thigh) flap (113), triceps flap (114), SIEA flap (115), TUG flap (116), and PAP flap (117). However, the abdominal wall remains the most popular donor site due to its consistent large volume and the added aesthetic benefit to the donor site, akin to an abdominoplasty. TRAM flaps have been successful at supplying adequate volume (118-120). However, they are associated with a significant number of partial flap necrosis (7%–31%) and donor site morbidity from subsequent rectus muscle weakness and ventral hernia (0.3%–11.0%) (121,122). As a result, muscle-sparing techniques have evolved and DIEP flaps have become popular. Despite being associated with longer microsurgical dissection and a substantial rate of fat necrosis, the introduction advanced modern imaging modalities, such as CTA, MRA, and FA, have improved flap outcomes in DIEP flaps (9,123). Since FA can only assess tissues up to 1 cm deep and a thick pannus is preferred in DIEP, it is less frequently utilized in the preoperative setting for perforator selection (8-10,125-129). Similarly, FA has a minimal role as a postoperative monitoring tool (12,42,48,130). Currently, FA has demonstrated most utility as an intraoperative tool to assess flap perfusion, microvascular anastomosis, and SIEA vascular territory.
Early studies have reported that ICG-indicated hypoperfused areas correlate accurately with areas of postoperative flap necrosis in TRAM and DIEP flaps (33-35,124,131-133). As a result, Newman et al. note that flap adjustments performed where FA demonstrated hypoperfusion led to successful reconstruction (34). In a larger retrospective analysis, Duggal et al. demonstrate a trend in the reduction of rate of flap necrosis (14% vs. 22%, P=0.237) and flap loss (1.4% vs. 3.4%, P=1.00) using ICG-guided excisions. Likewise, FA enables direct visualization of flow defect that may indicate anastomotic or vascular compromise in the early stages (134,135). Holm et al. report that FA can detect thrombotic occlusions with sensitivity of 100% and specificity of 86% (136). The same group calculated the time taken for the dye to travel from the arterial anastomosis to the venous anastomosis, and ITT of 50 seconds or greater would indicate postoperative re-exploration or flap loss with sensitivity of 92% and specificity of 98% (137). Despite being an attractive abdominal wall free flap option due to having no donor site morbidity, SIEA flap has been less commonly utilized due to its inconsistent vascular anatomy and variable size of its vascular territory (138,139). Encouraged by recent cadaveric and clinical studies suggesting that SIEA may be present more consistently (115,140-144), investigators have devised various management algorithms using laser Doppler flowmetry or ICG-based FA to reliably determine the vascular territory of SIEA for planning breast reconstruction (142,143,146). Currently, more evidences are still required to validate these algorithms.
Conclusions
For decades, FA has used for ophthalmic angiography, cardiothoracic surgery, hepatobilliary surgery, and neurosurgery for assessment of flow and perfusion and currently numerous commercial ICG detector systems are available. In plastic surgery and breast reconstruction, ICG-based FA is a relatively novel imaging technology shown promise in various applications. During sentinel lymph node biopsy, ICG has demonstrated a safer and a more accurate tracer agent, in lieu of the traditional blue dyes, for use with radioactive isotope. Using FA to guide excision of hypoperfused areas in implant-based breast reconstruction for high-risk patients—smoking, high BMI, and large breasts—has shown to improve clinical outcomes and be cost-effective. In autologous reconstructions, FA displays most promise in the intraoperative assessment of the flap perfusion, microvascular anastomosis, and SIEA flap.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.DeSantis C, Ma J, Bryan L, et al. Breast cancer statistics, 2013. CA Cancer J Clin 2014;64:52-62. [DOI] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer. GLOBOCAN 2008: Cancer Incidence and Mortality Worldwide. Accessed February 1, 2015. Available online: http://www.iarc.fr/en/media-centre/iarcnews/2010/globocan2008.php
- 3.Shons AR, Mosiello G. Postmastectomy breast reconstruction: current techniques. Cancer Control 2001;8:419-26. [DOI] [PubMed] [Google Scholar]
- 4.Lucas DJ, Sabino J, Shriver CD, et al. Doing more: trends in breast cancer surgery, 2005 to 2011. Am Surg 2015;81:74-80. [PubMed] [Google Scholar]
- 5.Blondeel PN. One hundred free DIEP flap breast reconstructions: a personal experience. Br J Plast Surg 1999;52:104-11. [DOI] [PubMed] [Google Scholar]
- 6.Healy C, Allen RJ, Sr. The evolution of perforator flap breast reconstruction: twenty years after the first DIEP flap. J Reconstr Microsurg 2014;30:121-5. [DOI] [PubMed] [Google Scholar]
- 7.Hamdi M, Khuthaila DK, Van Landuyt K, et al. Double-pedicle abdominal perforator free flaps for unilateral breast reconstruction: new horizons in microsurgical tissue transfer to the breast. J Plast Reconstr Aesthet Surg 2007;60:904-12; discussion 913-4. [DOI] [PubMed] [Google Scholar]
- 8.Chae MP, Hunter-Smith DJ, Rozen WM. Comparative analysis of fluorescent angiography, computed tomographic angiography and magnetic resonance angiography for planning autologous breast reconstruction. Gland Surg 2015;4:164-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rozen WM, Anavekar NS, Ashton MW, et al. Does the preoperative imaging of perforators with CT angiography improve operative outcomes in breast reconstruction? Microsurgery 2008;28:516-23. [DOI] [PubMed] [Google Scholar]
- 10.Masia J, Kosutic D, Cervelli D, et al. In search of the ideal method in perforator mapping: noncontrast magnetic resonance imaging. J Reconstr Microsurg 2010;26:29-35. [DOI] [PubMed] [Google Scholar]
- 11.Benson RC, Kues HA. Fluorescence properties of indocyanine green as related to angiography. Phys Med Biol 1978;23:159-63. [DOI] [PubMed] [Google Scholar]
- 12.Eren S, Rübben A, Krein R, et al. Assessment of microcirculation of an axial skin flap using indocyanine green fluorescence angiography. Plast Reconstr Surg 1995;96:1636-49. [DOI] [PubMed] [Google Scholar]
- 13.Jia Y, Bailey ST, Hwang TS, et al. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc Natl Acad Sci U S A 2015;112:E2395-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campagnoli TR, Medina CA, Singh AD. Choroidal melanoma initially treated as hemangioma: diagnostic and therapeutic considerations. Retin Cases Brief Rep 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15.Ehrlich P. Uber provozierte fluorescenz serscheinungen am auge. Deutsch Med Wochnschr 1882;8:35-6. [Google Scholar]
- 16.Centers for Medicare and Medicaid Services (CMS) , HHS. Medicare program; changes to the hospital inpatient prospective payment system for acute care hospitals and fiscal year 2010 rates; and changes to the long-term care hospital prospective payment system and rate years 2010 and 2009 rates. Final rules and interim final rule with comment period. Fed Regist 2009;74:43753-4236. [PubMed] [Google Scholar]
- 17.Handa T, Katare RG, Sasaguri S, et al. Preliminary experience for the evaluation of the intraoperative graft patency with real color charge-coupled device camera system: an advanced device for simultaneous capturing of color and near-infrared images during coronary artery bypass graft. Interact Cardiovasc Thorac Surg 2009;9:150-4. [DOI] [PubMed] [Google Scholar]
- 18.Aoki T, Yasuda D, Shimizu Y, et al. Image-guided liver mapping using fluorescence navigation system with indocyanine green for anatomical hepatic resection. World J Surg 2008;32:1763-7. [DOI] [PubMed] [Google Scholar]
- 19.Ishizawa T, Tamura S, Masuda K, et al. Intraoperative fluorescent cholangiography using indocyanine green: a biliary road map for safe surgery. J Am Coll Surg 2009;208:e1-4. [DOI] [PubMed] [Google Scholar]
- 20.Woitzik J, Horn P, Vajkoczy P, et al. Intraoperative control of extracranial-intracranial bypass patency by near-infrared indocyanine green videoangiography. J Neurosurg 2005;102:692-8. [DOI] [PubMed] [Google Scholar]
- 21.Losken A, Styblo TM, Schaefer TG, et al. The use of fluorescein dye as a predictor of mastectomy skin flap viability following autologous tissue reconstruction. Ann Plast Surg 2008;61:24-9. [DOI] [PubMed] [Google Scholar]
- 22.Graham BH, Walton RL, Elings VB, et al. Surface quantification of injected fluorescein as a predictor of flap viability. Plast Reconstr Surg 1983;71:826-33. [DOI] [PubMed] [Google Scholar]
- 23.Johnson RN, McDonald HR, Schatz H. Rash, fever, and chills after intravenous fluorescein angiography. Am J Ophthalmol 1998;126:837-8. [DOI] [PubMed] [Google Scholar]
- 24.Speich R, Saesseli B, Hoffmann U, et al. Anaphylactoid reactions after indocyanine-green administration. Ann Intern Med 1988;109:345-6. [DOI] [PubMed] [Google Scholar]
- 25.Hope-Ross M, Yannuzzi LA, Gragoudas ES, et al. Adverse reactions due to indocyanine green. Ophthalmology 1994;101:529-33. [DOI] [PubMed] [Google Scholar]
- 26.Benya R, Quintana J, Brundage B. Adverse reactions to indocyanine green: a case report and a review of the literature. Cathet Cardiovasc Diagn 1989;17:231-3. [DOI] [PubMed] [Google Scholar]
- 27.O'goshi K, Serup J. Safety of sodium fluorescein for in vivo study of skin. Skin Res Technol 2006;12:155-61. [DOI] [PubMed] [Google Scholar]
- 28.Myers B, Donovan W. An evaluation of eight methods of using fluorescein to predict the viability of skin flaps in the pig. Plast Reconstr Surg 1985;75:245-50. [DOI] [PubMed] [Google Scholar]
- 29.Kuebler WM, Sckell A, Habler O, et al. Noninvasive measurement of regional cerebral blood flow by near-infrared spectroscopy and indocyanine green. J Cereb Blood Flow Metab 1998;18:445-56. [DOI] [PubMed] [Google Scholar]
- 30.US Food and Drug Administration. IC-GREEN. Volume 20152015. Available online: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/
- 31.Hirche C, Murawa D, Mohr Z, et al. ICG fluorescence-guided sentinel node biopsy for axillary nodal staging in breast cancer. Breast Cancer Res Treat 2010;121:373-8. [DOI] [PubMed] [Google Scholar]
- 32.Antony AK, Mehrara BM, McCarthy CM, et al. Salvage of tissue expander in the setting of mastectomy flap necrosis: a 13-year experience using timed excision with continued expansion. Plast Reconstr Surg 2009;124:356-63. [DOI] [PubMed] [Google Scholar]
- 33.Komorowska-Timek E, Gurtner GC. Intraoperative perfusion mapping with laser-assisted indocyanine green imaging can predict and prevent complications in immediate breast reconstruction. Plast Reconstr Surg 2010;125:1065-73. [DOI] [PubMed] [Google Scholar]
- 34.Newman MI, Samson MC. The application of laser-assisted indocyanine green fluorescent dye angiography in microsurgical breast reconstruction. J Reconstr Microsurg 2009;25:21-6. [DOI] [PubMed] [Google Scholar]
- 35.Pestana IA, Coan B, Erdmann D, et al. Early experience with fluorescent angiography in free-tissue transfer reconstruction. Plast Reconstr Surg 2009;123:1239-44. [DOI] [PubMed] [Google Scholar]
- 36.Murray JD, Jones GE, Elwood ET, et al. Fluorescent intraoperative tissue angiography with indocyanine green: evaluation of nipple-areola vascularity during breast reduction surgery. Plast Reconstr Surg 2010;126:33e-34e. [DOI] [PubMed] [Google Scholar]
- 37.Brunworth LS, Samson MC, Newman MI, et al. Nipple-areola complex evaluation in long pedicled breast reductions with real-time fluorescent videoangiography. Plast Reconstr Surg 2011;128:585-6; author reply 586-7. [DOI] [PubMed] [Google Scholar]
- 38.Azuma R, Morimoto Y, Masumoto K, et al. Detection of skin perforators by indocyanine green fluorescence nearly infrared angiography. Plast Reconstr Surg 2008;122:1062-7. [DOI] [PubMed] [Google Scholar]
- 39.Lee BT, Matsui A, Hutteman M, et al. Intraoperative near-infrared fluorescence imaging in perforator flap reconstruction: current research and early clinical experience. J Reconstr Microsurg 2010;26:59-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsui A, Lee BT, Winer JH, et al. Quantitative assessment of perfusion and vascular compromise in perforator flaps using a near-infrared fluorescence-guided imaging system. Plast Reconstr Surg 2009;124:451-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bigdeli AK, Gazyakan E, Schmidt VJ, et al. Indocyanine Green Fluorescence for Free-Flap Perfusion Imaging Revisited: Advanced Decision Making by Virtual Perfusion Reality in Visionsense Fusion Imaging Angiography. Surg Innov 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 42.Mothes H, Dönicke T, Friedel R, et al. Indocyanine-green fluorescence video angiography used clinically to evaluate tissue perfusion in microsurgery. J Trauma 2004;57:1018-24. [DOI] [PubMed] [Google Scholar]
- 43.Holm C, Mayr M, Tegeler J, et al. Laser-induced fluorescence of indocyanine green: plastic surgical applications. Eur J Plast Surg 2003;26:19-25. [Google Scholar]
- 44.von Baeyer A. Ueber eine neue Klasse von Farbstoffen. Ber Dtsch Chem Ges 1871;4:555-8. [Google Scholar]
- 45.Zahr KA, Sherman JE, Chaglassian T, et al. Prediction of skin viability following en bloc resection for osteogenic sarcoma with fluorescein. Clin Orthop Relat Res 1983;(180):287-90. [PubMed] [Google Scholar]
- 46.Dibbell DG, Hedberg JR, McCraw JB, et al. A quantitative examination of the use of fluorescein in predicting viability of skin flaps. Ann Plast Surg 1979;3:101-5. [DOI] [PubMed] [Google Scholar]
- 47.Lange K, Boyd LJ. The use of fluorescein to determine the adequacy of the circulation. Med Clin North Am 1942;26:866. [Google Scholar]
- 48.Silverman DG, Norton KJ, Brousseau DA. Serial fluorometric documentation of fluorescein dye delivery. Surgery 1985;97:185-93. [PubMed] [Google Scholar]
- 49.Kogure K, David NJ, Yamanouchi U, et al. Infrared absorption angiography of the fundus circulation. Arch Ophthalmol 1970;83:209-14. [DOI] [PubMed] [Google Scholar]
- 50.Meijer DK, Weert B, Vermeer GA. Pharmacokinetics of biliary excretion in man. VI. Indocyanine green. Eur J Clin Pharmacol 1988;35:295-303. [DOI] [PubMed] [Google Scholar]
- 51.Administration UFaD. 510(k) Summary SPY Fluorescent Imaging System. Volume 20152007. Available online: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/
- 52.Nakayama A, del Monte F, Hajjar RJ, et al. Functional near-infrared fluorescence imaging for cardiac surgery and targeted gene therapy. Mol Imaging 2002;1:365-77. [DOI] [PubMed] [Google Scholar]
- 53.Electronic Radiology Lab. eXtensible Imaging Platform. Volume 20152009. Available online: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/
- 54.Troyan SL, Kianzad V, Gibbs-Strauss SL, et al. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol 2009;16:2943-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ballardini B, Santoro L, Sangalli C, et al. The indocyanine green method is equivalent to the 99mTc-labeled radiotracer method for identifying the sentinel node in breast cancer: a concordance and validation study. Eur J Surg Oncol 2013;39:1332-6. [DOI] [PubMed] [Google Scholar]
- 56.Pitsinis V, Provenzano E, Kaklamanis L, et al. Indocyanine green fluorescence mapping for sentinel lymph node biopsy in early breast cancer. Surg Oncol 2015;24:375-9. [DOI] [PubMed] [Google Scholar]
- 57.Tagaya N, Aoyagi H, Nakagawa A, et al. A novel approach for sentinel lymph node identification using fluorescence imaging and image overlay navigation surgery in patients with breast cancer. World J Surg 2011;35:154-8. [DOI] [PubMed] [Google Scholar]
- 58.Tanaka K, Okazaki M, Yano T, et al. Quantitative evaluation of blood perfusion to nerves included in the anterolateral thigh flap using indocyanine green fluorescence angiography: a different contrast pattern between the vastus lateralis motor nerve and femoral cutaneous nerve. J Reconstr Microsurg 2015;31:163-70. [DOI] [PubMed] [Google Scholar]
- 59.Wishart GC, Loh SW, Jones L, et al. A feasibility study (ICG-10) of indocyanine green (ICG) fluorescence mapping for sentinel lymph node detection in early breast cancer. Eur J Surg Oncol 2012;38:651-6. [DOI] [PubMed] [Google Scholar]
- 60.Hirche C, Engel H, Kolios L, et al. An experimental study to evaluate the Fluobeam 800 imaging system for fluorescence-guided lymphatic imaging and sentinel node biopsy. Surg Innov 2013;20:516-23. [DOI] [PubMed] [Google Scholar]
- 61.Swanson KI, Clark PA, Zhang RR, et al. Fluorescent cancer-selective alkylphosphocholine analogs for intraoperative glioma detection. Neurosurgery 2015;76:115-23; discussion 123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deming DA, Maher ME, Leystra AA, et al. Phospholipid ether analogs for the detection of colorectal tumors. PLoS One 2014;9:e109668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Antakia R, Gayet P, Guillermet S, et al. Near infrared fluorescence imaging of rabbit thyroid and parathyroid glands. J Surg Res 2014;192:480-6. [DOI] [PubMed] [Google Scholar]
- 64.Toens C, Krones CJ, Blum U, et al. Validation of IC-VIEW fluorescence videography in a rabbit model of mesenteric ischaemia and reperfusion. Int J Colorectal Dis 2006;21:332-8. [DOI] [PubMed] [Google Scholar]
- 65.Grommes J, Binnebösel M, Klink CD, et al. Comparison of intestinal microcirculation and wound healing in a rat model. J Invest Surg 2013;26:46-52. [DOI] [PubMed] [Google Scholar]
- 66.Hoffmann C, Compton F, Schäfer JH, et al. Intraoperative assessment of kidney allograft perfusion by laser-assisted indocyanine green fluorescence videography. Transplant Proc 2010;42:1526-30. [DOI] [PubMed] [Google Scholar]
- 67.Holm C, Mayr M, Höfter E, et al. Intraoperative evaluation of skin-flap viability using laser-induced fluorescence of indocyanine green. Br J Plast Surg 2002;55:635-44. [DOI] [PubMed] [Google Scholar]
- 68.Holm C, Tegeler J, Mayr M, et al. Monitoring free flaps using laser-induced fluorescence of indocyanine green: a preliminary experience. Microsurgery 2002;22:278-87. [DOI] [PubMed] [Google Scholar]
- 69.Krishnan KG, Schackert G, Steinmeier R. Near-infrared angiography and prediction of postoperative complications in various types of integumentary flaps. Plast Reconstr Surg 2004;114:1361-2. [DOI] [PubMed] [Google Scholar]
- 70.Murawa D, Hirche C, Dresel S, et al. Sentinel lymph node biopsy in breast cancer guided by indocyanine green fluorescence. Br J Surg 2009;96:1289-94. [DOI] [PubMed] [Google Scholar]
- 71.Sugie T, Kassim KA, Takeuchi M, et al. A novel method for sentinel lymph node biopsy by indocyanine green fluorescence technique in breast cancer. Cancers (Basel) 2010;2:713-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Noguchi M, Yokoi M, Nakano Y. Axillary reverse mapping with indocyanine fluorescence imaging in patients with breast cancer. J Surg Oncol 2010;101:217-21. [DOI] [PubMed] [Google Scholar]
- 73.Heitland AS, Markowicz MP, Koellensperger E, et al. Early and long-term evaluation of perfusion changes in free DIEP-flaps for breast reconstruction via IC-view and duplex ultrasound: autonomous or peripheral perfusion? J Reconstr Microsurg 2009;25:139-45. [DOI] [PubMed] [Google Scholar]
- 74.Benson JR. An alternative to initial axillary-lymph-node dissection. Lancet Oncol 2010;11:908-9. [DOI] [PubMed] [Google Scholar]
- 75.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst 2006;98:599-609. [DOI] [PubMed] [Google Scholar]
- 76.Purushotham AD, Upponi S, Klevesath MB, et al. Morbidity after sentinel lymph node biopsy in primary breast cancer: results from a randomized controlled trial. J Clin Oncol 2005;23:4312-21. [DOI] [PubMed] [Google Scholar]
- 77.Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol 2005;23:7703-20. [DOI] [PubMed] [Google Scholar]
- 78.Kitai T, Inomoto T, Miwa M, et al. Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer 2005;12:211-5. [DOI] [PubMed] [Google Scholar]
- 79.Benson J. Indocyanine Green Fluorescence for Sentinel Lymph Node Detection in Early Breast Cancer. Ann Surg Oncol 2016;23:6-8. [DOI] [PubMed] [Google Scholar]
- 80.Sugie T, Kinoshita T, Masuda N, et al. Evaluation of the Clinical Utility of the ICG Fluorescence Method Compared with the Radioisotope Method for Sentinel Lymph Node Biopsy in Breast Cancer. Ann Surg Oncol 2016;23:44-50. [DOI] [PubMed] [Google Scholar]
- 81.Samorani D, Fogacci T, Panzini I, et al. The use of indocyanine green to detect sentinel nodes in breast cancer: a prospective study. Eur J Surg Oncol 2015;41:64-70. [DOI] [PubMed] [Google Scholar]
- 82.Verbeek FP, Troyan SL, Mieog JS, et al. Near-infrared fluorescence sentinel lymph node mapping in breast cancer: a multicenter experience. Breast Cancer Res Treat 2014;143:333-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ahmed M, Purushotham AD, Douek M. Novel techniques for sentinel lymph node biopsy in breast cancer: a systematic review. Lancet Oncol 2014;15:e351-62. [DOI] [PubMed] [Google Scholar]
- 84.Onishi K, Maruyama Y, Iwahira Y. Cutaneous and fascial vasculature of the leg: anatomic study of fasciocutaneous vessels. J Reconstr Microsurg 1986;2:181-9. [DOI] [PubMed] [Google Scholar]
- 85.Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. Breast reconstruction: increasing implant rates. Plast Reconstr Surg 2013;131:15-23. [DOI] [PubMed] [Google Scholar]
- 86.Selber JC, Wren JH, Garvey PB, et al. Critical Evaluation of Risk Factors and Early Complications in 564 Consecutive Two-Stage Implant-Based Breast Reconstructions Using Acellular Dermal Matrix at a Single Center. Plast Reconstr Surg 2015;136:10-20. [DOI] [PubMed] [Google Scholar]
- 87.Breuing KH, Bayer L, Neuwalder J, et al. Early experience using low-frequency ultrasound in chronic wounds. Ann Plast Surg 2005;55:183-7. [DOI] [PubMed] [Google Scholar]
- 88.Salzberg CA. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann Plast Surg 2006;57:1-5. [DOI] [PubMed] [Google Scholar]
- 89.Zienowicz RJ, Karacaoglu E. Implant-based breast reconstruction with allograft. Plast Reconstr Surg 2007;120:373-81. [DOI] [PubMed] [Google Scholar]
- 90.Krishnan NM, Chatterjee A, Van Vliet MM, et al. A comparison of acellular dermal matrix to autologous dermal flaps in single-stage, implant-based immediate breast reconstruction: a cost-effectiveness analysis. Plast Reconstr Surg 2013;131:953-61. [DOI] [PubMed] [Google Scholar]
- 91.Johnson RK, Wright CK, Gandhi A, et al. Cost minimisation analysis of using acellular dermal matrix (Strattice™) for breast reconstruction compared with standard techniques. Eur J Surg Oncol 2013;39:242-7. [DOI] [PubMed] [Google Scholar]
- 92.Davila AA, Mioton LM, Chow G, et al. Immediate two-stage tissue expander breast reconstruction compared with one-stage permanent implant breast reconstruction: a multi-institutional comparison of short-term complications. J Plast Surg Hand Surg 2013;47:344-9. [DOI] [PubMed] [Google Scholar]
- 93.Phillips BT, Lanier ST, Conkling N, et al. Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: results of a prospective trial. Plast Reconstr Surg 2012;129:778e-88e. [DOI] [PubMed] [Google Scholar]
- 94.Duggal CS, Madni T, Losken A. An outcome analysis of intraoperative angiography for postmastectomy breast reconstruction. Aesthet Surg J 2014;34:61-5. [DOI] [PubMed] [Google Scholar]
- 95.American Society of Plastic Surgeons. ASPS National Clearinghouse of plastic surgery procedural statistics. Available online: http://www.plasticsurgery.org/Documents/news-resources/statistics/2013-statistics/plastic-surgery-statistics-full-report-2013.pdf
- 96.Alderman AK, Wilkins EG, Kim HM, et al. Complications in postmastectomy breast reconstruction: two-year results of the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg 2002;109:2265-74. [DOI] [PubMed] [Google Scholar]
- 97.Sullivan SR, Fletcher DR, Isom CD, et al. True incidence of all complications following immediate and delayed breast reconstruction. Plast Reconstr Surg 2008;122:19-28. [DOI] [PubMed] [Google Scholar]
- 98.Kim JY, Davila AA, Persing S, et al. A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction. Plast Reconstr Surg 2012;129:28-41. [DOI] [PubMed] [Google Scholar]
- 99.Kanuri A, Liu AS, Guo L. Whom should we SPY? A cost analysis of laser-assisted indocyanine green angiography in prevention of mastectomy skin flap necrosis during prosthesis-based breast reconstruction. Plast Reconstr Surg 2014;133:448e-54e. [DOI] [PubMed] [Google Scholar]
- 100.Moyer HR, Losken A. Predicting mastectomy skin flap necrosis with indocyanine green angiography: the gray area defined. Plast Reconstr Surg 2012;129:1043-8. [DOI] [PubMed] [Google Scholar]
- 101.Munabi NC, Olorunnipa OB, Goltsman D, et al. The ability of intra-operative perfusion mapping with laser-assisted indocyanine green angiography to predict mastectomy flap necrosis in breast reconstruction: a prospective trial. J Plast Reconstr Aesthet Surg 2014;67:449-55. [DOI] [PubMed] [Google Scholar]
- 102.Newman MI, Samson MC, Tamburrino JF, et al. Intraoperative laser-assisted indocyanine green angiography for the evaluation of mastectomy flaps in immediate breast reconstruction. J Reconstr Microsurg 2010;26:487-92. [DOI] [PubMed] [Google Scholar]
- 103.Singer R, Lewis CM, Franklin JD, et al. Fluorescein test for prediction of flap viability during breast reconstructions. Plast Reconstr Surg 1978;61:371-5. [DOI] [PubMed] [Google Scholar]
- 104.Thomson JG, Kerrigan CL. Dermofluorometry: thresholds for predicting flap survival. Plast Reconstr Surg 1989;83:859-64; discussion 865. [PubMed] [Google Scholar]
- 105.Proano E, Perbeck LG. Influence of the site of skin incision on the circulation in the nipple-areola complex after subcutaneous mastectomy in breast cancer. Scand J Plast Reconstr Surg Hand Surg 1996;30:195-200. [DOI] [PubMed] [Google Scholar]
- 106.De Lorenzi F, Yamaguchi S, Petit JY, et al. Evaluation of skin perfusion after nipple-sparing mastectomy by indocyanine green dye. Preliminary results. J Exp Clin Cancer Res 2005;24:347-54. [PubMed] [Google Scholar]
- 107.Kroll SS. Why autologous tissue? Clin Plast Surg. 1998;25:135-43. [PubMed] [Google Scholar]
- 108.Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg 1994;32:32-8. [DOI] [PubMed] [Google Scholar]
- 109.Kiricuta I. The use of the great omentum in the surgery of breast cancer. Presse Med 1963;71:15-7. [PubMed] [Google Scholar]
- 110.Fujino T, Harasina T, Aoyagi F. Reconstruction for aplasia of the breast and pectoral region by microvascular transfer of a free flap from the buttock. Plast Reconstr Surg 1975;56:178-81. [DOI] [PubMed] [Google Scholar]
- 111.Olivari N. The latissimus flap. Br J Plast Surg 1976;29:126-8. [DOI] [PubMed] [Google Scholar]
- 112.Serafin D, Georgiade NG. Transfer of free flaps to provide well-vascularized, thick cover for breast reconstructions after radical mastectomy. Plast Reconstr Surg 1978;62:527-36. [DOI] [PubMed] [Google Scholar]
- 113.Elliott LF, Beegle PH, Hartrampf CR, Jr. The lateral transverse thigh free flap: an alternative for autogenous-tissue breast reconstruction. Plast Reconstr Surg 1990;85:169-78; discussion 179-81. [PubMed] [Google Scholar]
- 114.Hartrampf CR, Jr, Elliott LF, Feldman S. A triceps musculocutaneous flap for chest-wall defects. Plast Reconstr Surg 1990;86:502-9. [DOI] [PubMed] [Google Scholar]
- 115.Arnez ZM, Khan U, Pogorelec D, et al. Breast reconstruction using the free superficial inferior epigastric artery (SIEA) flap. Br J Plast Surg 1999;52:276-9. [DOI] [PubMed] [Google Scholar]
- 116.Peek A, Müller M, Exner K. The free gracilis perforator flap for autologous breast reconstruction. Handchir Mikrochir Plast Chir 2002;34:245-50. [DOI] [PubMed] [Google Scholar]
- 117.Allen RJ, Haddock NT, Ahn CY, et al. Breast reconstruction with the profunda artery perforator flap. Plast Reconstr Surg 2012;129:16e-23e. [DOI] [PubMed] [Google Scholar]
- 118.Robbins TH. Rectus abdominis myocutaneous flap for breast reconstruction. Aust N Z J Surg 1979;49:527-30. [DOI] [PubMed] [Google Scholar]
- 119.Holmström H. The free abdominoplasty flap and its use in breast reconstruction. An experimental study and clinical case report. Scand J Plast Reconstr Surg 1979;13:423-27. [DOI] [PubMed] [Google Scholar]
- 120.Hartrampf CR, Scheflan M, Black PW. Breast reconstruction with a transverse abdominal island flap. Plast Reconstr Surg 1982;69:216-25. [DOI] [PubMed] [Google Scholar]
- 121.Nahabedian MY, Momen B, Galdino G, et al. Breast Reconstruction with the free TRAM or DIEP flap: patient selection, choice of flap, and outcome. Plast Reconstr Surg 2002;110:466-75; discussion 476-7. [DOI] [PubMed] [Google Scholar]
- 122.Nahabedian MY, Tsangaris T, Momen B. Breast reconstruction with the DIEP flap or the muscle-sparing (MS-2) free TRAM flap: is there a difference? Plast Reconstr Surg 2005;115:436-44; discussion 445-6. [DOI] [PubMed] [Google Scholar]
- 123.Mathes DW, Neligan PC. Current techniques in preoperative imaging for abdomen-based perforator flap microsurgical breast reconstruction. J Reconstr Microsurg 2010;26:3-10. [DOI] [PubMed] [Google Scholar]
- 124.Francisco BS, Kerr-Valentic MA, Agarwal JP. Laser-assisted indocyanine green angiography and DIEP breast reconstruction. Plast Reconstr Surg 2010;125:116e-8e. [DOI] [PubMed] [Google Scholar]
- 125.Pestana IA, Zenn MR. Correlation between abdominal perforator vessels identified with preoperative CT angiography and intraoperative fluorescent angiography in the microsurgical breast reconstruction patient. Ann Plast Surg 2014;72:S144-9. [DOI] [PubMed] [Google Scholar]
- 126.Rozen WM, Chubb D, Grinsell D, et al. Computed tomographic angiography: clinical applications. Clin Plast Surg 2011;38:229-39. [DOI] [PubMed] [Google Scholar]
- 127.Masia J, Clavero JA, Larrañaga JR, et al. Multidetector-row computed tomography in the planning of abdominal perforator flaps. J Plast Reconstr Aesthet Surg 2006;59:594-9. [DOI] [PubMed] [Google Scholar]
- 128.Masia J, Kosutic D, Clavero JA, et al. Preoperative computed tomographic angiogram for deep inferior epigastric artery perforator flap breast reconstruction. J Reconstr Microsurg 2010;26:21-8. [DOI] [PubMed] [Google Scholar]
- 129.Rozen WM, Stella DL, Bowden J, et al. Advances in the pre-operative planning of deep inferior epigastric artery perforator flaps: magnetic resonance angiography. Microsurgery 2009;29:119-23. [DOI] [PubMed] [Google Scholar]
- 130.Rübben A, Eren S, Krein R, et al. Infrared videoangiofluorography of the skin with indocyanine green--rat random cutaneous flap model and results in man. Microvasc Res 1994;47:240-51. [DOI] [PubMed] [Google Scholar]
- 131.Wu C, Kim S, Halvorson EG. Laser-assisted indocyanine green angiography: a critical appraisal. Ann Plast Surg 2013;70:613-9. [DOI] [PubMed] [Google Scholar]
- 132.Jones GE, Garcia CA, Murray J, et al. Fluorescent intraoperative tissue angiography for the evaluation of the viability of pedicled TRAM flaps. Plast Reconstr Surg 2009;124:53.19568044 [Google Scholar]
- 133.Yamaguchi S, De Lorenzi F, Petit JY, et al. The "perfusion map" of the unipedicled TRAM flap to reduce postoperative partial necrosis. Ann Plast Surg 2004;53:205-9. [DOI] [PubMed] [Google Scholar]
- 134.Liu DZ, Mathes DW, Zenn MR, et al. The application of indocyanine green fluorescence angiography in plastic surgery. J Reconstr Microsurg 2011;27:355-64. [DOI] [PubMed] [Google Scholar]
- 135.Holm C, Mayr M, Höfter E, et al. Assessment of the patency of microvascular anastomoses using microscope-integrated near-infrared angiography: a preliminary study. Microsurgery 2009;29:509-14. [DOI] [PubMed] [Google Scholar]
- 136.Holm C, Dornseifer U, Sturtz G, et al. Sensitivity and specificity of ICG angiography in free flap reexploration. J Reconstr Microsurg 2010;26:311-6. [DOI] [PubMed] [Google Scholar]
- 137.Holm C, Dornseifer U, Sturtz G, et al. The intrinsic transit time of free microvascular flaps: clinical and prognostic implications. Microsurgery 2010;30:91-6. [DOI] [PubMed] [Google Scholar]
- 138.Tregaskiss A. Perfusion zones of the DIEP flap revisited: a clinical study. Plast Reconstr Surg 2006;118:816; author reply 816-7. [DOI] [PubMed] [Google Scholar]
- 139.Taylor GI, Daniel RK. The anatomy of several free flap donor sites. Plast Reconstr Surg 1975;56:243-53. [DOI] [PubMed] [Google Scholar]
- 140.Reardon CM, O'Ceallaigh S, O'Sullivan ST. An anatomical study of the superficial inferior epigastric vessels in humans. Br J Plast Surg 2004;57:515-9. [DOI] [PubMed] [Google Scholar]
- 141.Chevray PM. Breast reconstruction with superficial inferior epigastric artery flaps: a prospective comparison with TRAM and DIEP flaps. Plast Reconstr Surg 2004;114:1077-83; discussion 1084-5. [DOI] [PubMed] [Google Scholar]
- 142.Ulusal BG, Cheng MH, Wei FC, et al. Breast reconstruction using the entire transverse abdominal adipocutaneous flap based on unilateral superficial or deep inferior epigastric vessels. Plast Reconstr Surg 2006;117:1395-403; discussion 1404-6. [DOI] [PubMed] [Google Scholar]
- 143.Holm C, Mayr M, Höfter E, et al. The versatility of the SIEA flap: a clinical assessment of the vascular territory of the superficial epigastric inferior artery. J Plast Reconstr Aesthet Surg 2007;60:946-51. [DOI] [PubMed] [Google Scholar]
- 144.Spiegel AJ, Khan FN. An Intraoperative algorithm for use of the SIEA flap for breast reconstruction. Plast Reconstr Surg 2007;120:1450-9. [DOI] [PubMed] [Google Scholar]
- 145.Wolfram D, Schoeller T, Hussl H, et al. The superficial inferior epigastric artery (SIEA) flap: indications for breast reconstruction. Ann Plast Surg 2006;57:593-6. [DOI] [PubMed] [Google Scholar]
- 146.Holm C, Mayr M, Höfter E, et al. Interindividual variability of the SIEA Angiosome: effects on operative strategies in breast reconstruction. Plast Reconstr Surg 2008;122:1612-20. [DOI] [PubMed] [Google Scholar]


