Abstract
Background
Computed tomographic (CT) angiography (CTA) is widely considered the gold standard imaging modality for preoperative planning autologous breast reconstruction with deep inferior epigastric artery (DIEA) perforator (DIEP) flap. Improved anatomical understanding from CTA has translated to enhanced clinical outcomes. To achieve this, the use of appropriate CT hardware and software is vital. Various CT scanners and contrast materials have been demonstrated to consistently produce adequate scan data. However, the availability of affordable and easily accessible imaging software capable of generating 3D volume-rendered perforator images to clinically useful quality has been lacking. Osirix (Pixmeo, Geneva, Switzerland) is a free, readily available medical image processing software that shows promise. We have previously demonstrated in a case report the usefulness of Osirix in localizing perforators and their course.
Methods
In the current case series of 50 consecutive CTA scans, we compare the accuracy of Osirix to a commonly used proprietary 3D imaging software, Siemens Syngo InSpace 4D (Siemens, Erlangen, Germany), in identifying perforator number and location. Moreover, we compared both programs to intraoperative findings.
Results
We report a high rate of concordance with Osirix and Siemens Syngo InSpace 4D (99.6%). Both programs correlated closely with operative findings (92.2%). Most of the discrepancies were found in the lateral row perforators (90%).
Conclusions
In the current study, we report the accuracy of Osirix that is comparable to Siemens Syngo InSpace 4D, a proprietary software, in mapping perforators. However, it provides an added advantage of being free, easy-to-use, portable, and potentially a superior quality of 3D reconstructed image.
Keywords: Computed tomographic angiography (CTA), perforator imaging, Osirix, Siemens, accuracy
Introduction
Currently, computed tomographic (CT) angiography (CTA) is widely considered the gold standard perforator imaging technique for preoperative planning an autologous breast reconstruction with deep inferior epigastric artery (DIEA) perforator (DIEP) flap (1,2). The scan data can be 3D reconstructed to produce a “perforator map” that assists surgeons in selecting an appropriate perforator, donor site, and the flap. A plethora of studies have demonstrated a high accuracy of CTA in detecting perforators, reporting a sensitivity and specificity close to 100% (3-12). In comparison to other perforator imaging modalities, such as Doppler ultrasound and magnetic resonance angiography (MRA), CTA has demonstrated superior visualization of the perforators and their subcutaneous course, respectively (7,10). While MRA may be evolving in this role, widespread outcome data is still lacking. The benefits of CTA have translated into improved clinical outcomes, such as increased flap survival, reduced donor site morbidity, and reduced operating time (5,6,10,12-24). To this end, appropriate use of hardware and software is essential to obtain optimal perforator data from CTA.
Through various scanner hardware brands (i.e., Siemens, Toshiba, and Philips), varying number of multi-detector rows (i.e., 4-slice to 320-slice scanners) and differing contrast media and volumes, all scanners and techniques are able to achieve high quality and clinically useful images (1,2). In addition, we have published optimized CTA scanning techniques that enhance perforator visualizations, such as initiating contrast bolus trigger at the common femoral artery, moving the computed tomography table caudo-cranially, and disabling the Siemens Care Dose 4D feature (10).
High cost and limited accessibility of 3D imaging softwares that generate 3D reconstructions suitable for clinical use have been challenging for hospitals with relatively limited resources. Most of the currently available proprietary softwares, such as Siemens Syngo InSpace 4D (Siemens, Erlangen, Germany) (25) and VoNaviX (IVS Technology, Chemnitz, Germany) (26) are expensive. Some are not readily accessible outside the institution where it was originally developed, such as virSSPA (University Hospitals Virgen del Rocio, Sevilla, Spain) (15). Furthermore, many programs available cannot provide adequate images, with some not able to visualize perforators to a clinically useful degree. One particular program that we have found that can achieve optimal images is Siemens Syngo InSpace 4D (10). The program enables users to assign color to various contrast values using color look-up table (CLUT) function, providing superior contrast resolution to the 3D reconstructions. Again however, the cost and availability are significant limitations. Previously, we have demonstrated the application of a free 3D imaging program, Osirix (Pixmeo, Geneva, Switzerland).
Osirix is a free imaging processing software, specifically designed for medical imaging by a radiologist, and is readily downloaded online for use unreservedly (27). It is able to produce the same or better images than the currently available programs on a user-friendly interface. Furthermore, Osirix can be readily operated on a laptop computer, which enables viewing in the operating theatre or at home. Similar to Siemens Syngo InSpace, Osirix enables the user to create 3D volume-rendered reconstructions and assign colors using an appropriate CLUT function to optimize visualization of perforators and their course, as demonstrated in our previous case report (28).
In the current study, we investigate the accuracy of the freely available 3D imaging software, Osirix, by comparing it to the proprietary program, Siemens Syngo InSpace 4D, and also comparing both softwares to the intraoperative findings.
Methods
The study design was a prospective case series. A total of 50 consecutive patients (i.e., 100 hemi-abdominal walls) underwent CTA prior to a DIEP flap breast reconstruction. All patients were aged between 30 and 60 years and spanned a wide range of body habitus. All imaging findings were recorded by a single operator and all intraoperative findings were recorded by the operating surgeon.
CTA technique
All scans were performed at a single institution (Future Medical Imaging Group, Melbourne, Australia) using a standardized protocol that has been modified and improved from the conventional CTA methodology in order to maximize the image quality and minimize radiation exposure (10,21). The computed tomography scanner used was a Siemens SOMATOM Sensation 64 multi-detector row computed tomography scanner (Siemens Medical Solutions, Erlangen, Germany) and the scan parameters are summarized in Table 1.
Table 1. Computed tomographic scan parameters.
| Parameters | |
|---|---|
| Scanner | Siemens SOMATOM Sensation 64 |
| Scan type | Helical multi detector row CT angiography |
| Slice thickness | 64 detector row ×0.6 mm collimator width |
| Helical detector pitch | 0.9 |
| Gantry rotation speed | 0.37 s |
| Tube potential | 120 kV |
| Tube current | 180 mA |
| Contrast | Omnipaque 350 100 mL IV injection 4 mL per second |
| Scanning range | Pubic symphysis to 4 cm above umbilicus |
| Scanning direction | Caudo-cranial |
| Bolus tracking | +100 HU from common femoral artery with minimal delay |
| Automatic dose modulation (Siemens Care Dose 4D) | Disabled |
| Imaging reconstruction | 1 mm/0.7 mm overlapping axial images |
CT, computed tomographic; HU, Hounsfield units.
Patients were scanned in a position matching operative positioning: supine, with no clothing or straps to deform the abdominal contour. The scan range was limited to the tissue used intraoperatively and thus spanned from the pubic symphysis to 4 cm above the level of umbilicus. A bolus of 100 mL of intravenous omnipaque 350 was used for contrast, without oral contrast. We have previously described three major modifications introduced to the standard CTA protocol in order to enhance the arterial phase filling and the resolution of cutaneous vasculature (10). Briefly, the contrast bolus trigger to begin scanning was taken at the common femoral artery; the computed tomography table movement was reversed to scan caudo-cranially from the pubic symphysis to match the filling of DIEA; and the Siemens Care Dose 4D features was disabled, which maximized the abdominal wall signal-to-noise ratio.
Scan analysis
CTA scans were analyzed using both imaging softwares: Siemens Syngo InSpace 4D (Version 2006A; Siemens, Erlangen, Germany) and Osirix (Pixmeo, Geneva, Switzerland). The thin-slice (i.e., 1 mm or less) axial raw data were reformatted into 3D volume-rendered reconstructions and maximum intensity projections (MIPs) to identify the number and location of perforators, and the branching pattern of DIEA (29).
Perforator mapping
3D-reconstructed images of the abdominal wall perforators are generated using volume-rendering technique (VRT) and MIP techniques. VRT reconstructions required the use of the CLUT function found in both of the image processing softwares. Additionally in Osirix (Pixmeo, Geneva, Switzerland), we applied Gaussian blur to the final 3D reconstruction facilitating the removal of interference within the data (Figures 1,2). All infraumbilical perforators with diameter greater than 0.5 mm were identified and mapped on VRT reconstructions. Arrowheads were placed at the point of emergence of each perforator from the anterior rectus sheath. They were overlaid on to a 2D representation of each patient’s abdominal wall with a grid of 4 mm squares applied to the image centered on the umbilicus as reference point. The transverse distances of each perforator from the midline were recorded to the closest 0.5 cm. The perforators were recorded as found in medial or lateral row. MIP reconstructions were used to illustrate intramuscular course of the perforators.
Figure 1.
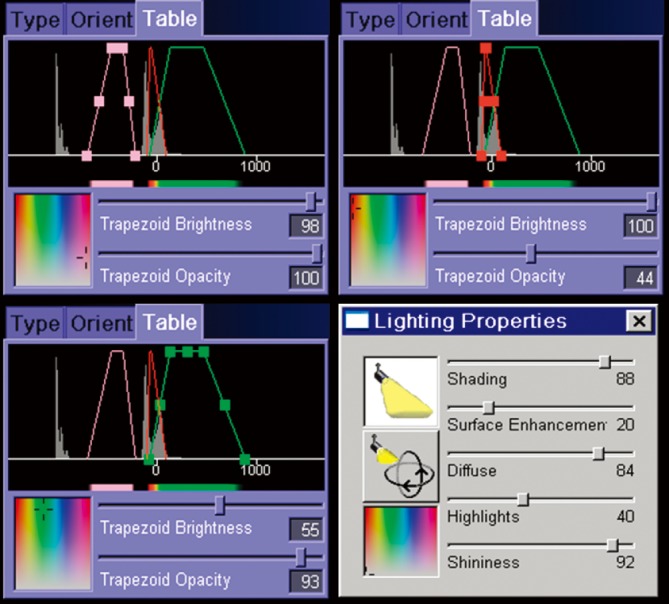
Color look-up table (CLUT) and ray cast lighting properties in Siemens Syngo InSpace 4D (Siemens, Erlangen, Germany). Reproduced with permission from Rozen et al. (25).
Figure 2.
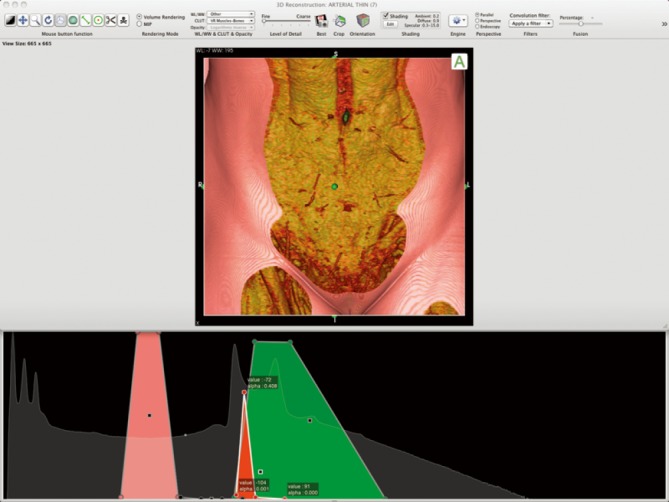
Color look-up table (CLUT) in Osirix (Pixmeo, Geneva, Switzerland), designed for perforator imaging. Reproduced with permission from Rozen et al. (28).
Intraoperative measurements
The perforator locations were compared with operative findings, where they were located on equivalent grids. Intraoperative grids were placed over the lower abdominal wall, with the umbilicus and midline as references, and the location of perforators was documented on it with sterile pens. A 0.5-cm margin of error was given for the location of each perforator. This was a conservative figure given as an estimate of the combined error associated with the calculation of concordance, and included the following factors: CTA error (e.g., patient movement, venous contamination), CTA reporting error (e.g., multiplanar reformatting error, reading error), intraoperative measurement error (e.g., limitation of measurement tool, reading error), and patient error (e.g., umbilical shift, abdominal pannus mobility). For the purpose of comparison, the operative findings were considered the standard.
All perforators were explored bilaterally, including the perforators not included in the flap. All perforators greater than 0.5 mm in diameter were included in the study and recorded in the manner described. As achieved during the CTA scan interpretation, the perforators identified intraoperatively comprised arterial perforators and not adjacent veins.
Statistical analysis
The perforator locations were recorded as exact values and the findings were compared between the two software programs. In addition, the data from each program was compared to the operative findings. The comparative analysis was conducted using SPSS Statistics software package (IBM, Armonk, New York, USA) and the outcomes were analyzed using paired Student’s t-test. A P value of <0.05 was accepted as statistically significant.
Results
A total of 50 CTA scans were performed in 50 consecutive cases (i.e., 100 hemi-abdominal walls) that identified 512 perforators of DIEA at an average of 5.12 perforators per hemi-abdomen. Concordance between Siemens Syngo InSpace 4D (version 2006A; Siemens, Erlangen, Germany) and Osirix (Pixmeo, Geneva, Switzerland) in accurately identifying perforator locations, and comparison between each of the software programs to intraoperative findings were evaluated.
Between Siemens Syngo InSpace 4D and Osirix, 510 out of 512 perforators (99.6%) had concordance. The two discordant perforators between the imaging programs were located in the lateral row and had only 0.5 cm of difference. Mean transverse distance from the midline using both software programs was 3.36 cm, with no statistical difference between them for measuring perforator location (Table 2 and Figure 3).
Table 2. Mean transverse distance of DIEA perforators from the midline as identified using the 3D imaging softwares: Siemens Syngo InSpace 4D (Siemens, Erlangen, Germany) and Osirix (Pixmeo, Geneva, Switzerland).
| Siemens Syngo InSpace 4D | Osirix | Difference | P value | |
|---|---|---|---|---|
| Perforator location, lateral-to-midline (mean) | 3.36 cm | 3.36 cm | 0 cm | 1 |
DIEA, deep inferior epigastric artery.
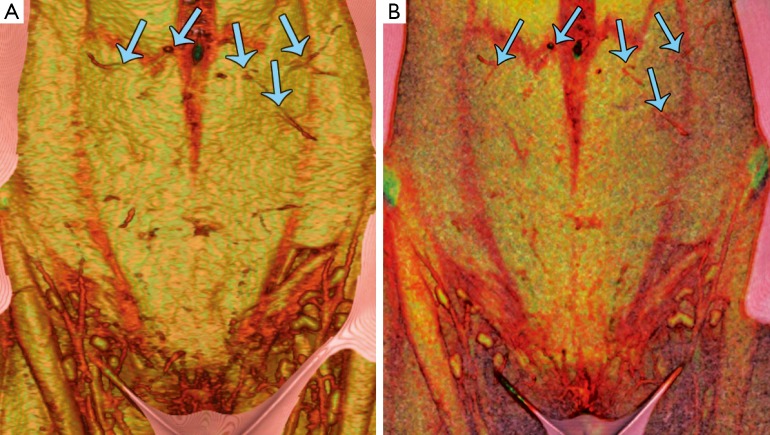
Figure 3.
Preoperative computed tomography angiography (CTA), volume-rendered reconstruction of the abdominal wall vasculature with: (A) Osirix (Pixmeo, Geneva, Switzerland); and (B) Siemens Syngo InSpace 4D (Siemens, Erlangen, Germany). Both techniques clearly demonstrate several large periumbilical perforators (blue arrows), and highlight features of the abdominal wall soft-tissues. Reproduced with permission from Rozen et al. (28).
Between each of the softwares and the operative findings, there was a mean difference of 0.7 mm per perforator using both programs (Tables 3,4). Although this difference was statistically significant (P<0.01), this was not a clinically significant difference (i.e., less than 1 mm).
Table 3. Comparing the mean transverse distance of DIEA perforators from the midline calculated using Siemens Syngo InSpace 4D (Siemens, Erlangen, Germany) to the intraoperative measurements.
| Siemens Syngo InSpace 4D | Operative findings | Difference | P value | |
|---|---|---|---|---|
| Perforator location, lateral-to-midline (mean) | 3.36 cm | 3.43 cm | 0.7 cm | <0.01 |
DIEA, deep inferior epigastric artery.
Table 4. Comparing the mean transverse distance of DIEA perforators from the midline calculated using Osirix (Pixmeo, Geneva, Switzerland) to the intraoperative measurements.
| Osirix | Operative findings | Difference | P value | |
|---|---|---|---|---|
| Perforator location, lateral-to-midline (mean) | 3.36 cm | 3.43 cm | 0.7 cm | <0.01 |
DIEA, deep inferior epigastric artery.
An analysis of perforators that had a difference between imaging and intraoperative findings was undertaken, with 40 perforators (7.8%) discordant between imaging and operative findings (Table 5). Of 18 perforators that had 0.5 cm difference with operative findings, 7 were located in medial row and 11 in lateral row. Of 12 perforators that had 1 cm difference, 5 were located in medial row and 7 in lateral row. Of 8 perforators that had 1.5 cm difference, 1 was located in medial row and 7 in lateral row. Of 2 perforators that had 2 cm difference, none were located in medial row and 2 in lateral row. Medial row perforators accounted for 13 out of 40 discordant results (32.5%) and lateral row 27 out of 40 (67.5%). Hence, imaging was more accurate when assessing medial row perforators (32.5% vs. 67.5%). Furthermore, when specifically assessing the larger discrepancies (>1 cm), medial row accounted for only 1 out of 10 (10%) and lateral row 9 out of 10 (90%).
Table 5. Analysis of discrepancy found in the perforator localization between imaging and operative findings and their distribution between medial and lateral rows.
| Medial row | Lateral row | Total | |
|---|---|---|---|
| Imaging: operative discrepancy 0.5 cm (number of perforators) | 7 | 11 | 18 |
| Imaging: operative discrepancy 1.0 cm (number of perforators) | 5 | 7 | 12 |
| Imaging: operative discrepancy 1.5 cm (number of perforators) | 1 | 7 | 8 |
| Imaging: operative discrepancy 2.0 cm (number of perforators) | 0 | 2 | 2 |
| Total | 13 | 27 | 40 |
Discussion
An improved understanding of the DIEA and its perforators from CTA has assisted reconstructive surgeons in the selection of the appropriate donor site, perforator, and hemi-abdominal wall of choice for reconstruction, which has translated to significant improvements in clinical outcomes (5,6,10,12-24). To achieve this, the use of appropriate hardware and software is vital. For CTA hardware, CT scanners from various brands using different multi-detector rows with varying IV contrast materials and volumes have demonstrated in the literature to deliver consistently sufficient scan data (1,3,5,10,12,15). In contrast, the high cost and limited accessibility of image processing software that can produce clinically useful 3D volume-rendered reconstructions have limited a wide application of CTA. To this effect, Osirix, a medical imaging program available for free online, have been useful. It is capable of producing the same or superior quality 3D reconstructions than the proprietary softwares and has added advantages of user-friendly interface and portability.
We have previously described the potential utility of Osirix for preoperatively planning a DIEP flap breast reconstruction in a case report (28). In the current case series, we demonstrate that Osirix is as accurate as the commonly used proprietary software, Siemens Syngo InSpace 4D, in identifying perforator number and location (99.6%). Furthermore, the measurements from both programs closely correlated to the operative findings (92.2%). The discordance between imaging and operative findings was most pronounced in assessing lateral row perforators (90% vs. 10%). For the purpose of the current study, we forewent comparing perforator diameters since these measurements can be made on standard axial slices of a CTA, regardless of the software program.
In addition to its accuracy in perforator localization, Osirix has the potential to yield superior quality 3D images than Siemens Syngo InSpace 4D due to its 16-bit CLUT function and the capacity to apply Gaussian blur after the 3D reconstruction to reduce interference. Furthermore, Osirix exhibits an easy-to-navigate user interface that is readily accessible to clinicians without technological background and it is compatible on Mac operating system. As a result, surgeons can access the 3D reconstructed images on their portable computer in the operating theatre or at home.
Of note, although free for the basic version, there is a cost to the fully functional version that allows more images to be processed and your own presets to be used. Even this version offers a widely affordable option for most institutions compared to other options.
One of the limitations of the current study is our relatively small sample size. A larger randomized study with greater sample size will be required to further validate our findings. Moreover, a future study may consider comparing Osirix to a host of other proprietary softwares, such as VoNaviX, and their impact on clinical outcomes. For the purpose of this study, the comparative analysis was performed in cases of autologous breast reconstruction with DIEP flap. However, validating Osirix in assessing other free flap options for autologous breast reconstruction may be of value.
Conclusions
This comparative analysis demonstrates that the accuracy of Osirix, a freely available medical image processing software, is concordant with Siemens Syngo InSpace 4D, a commonly utilized proprietary software, in localizing perforators for autologous breast reconstruction with DIEP flaps. Measurements from both programs correlated equally to the intraoperative findings. Most of the discrepancies arose in the lateral row perforators.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Chae MP, Hunter-Smith DJ, Rozen WM. Comparative analysis of fluorescent angiography, computed tomographic angiography and magnetic resonance angiography for planning autologous breast reconstruction. Gland Surg 2015;4:164-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rozen WM, Chubb D, Grinsell D, et al. Computed tomographic angiography: clinical applications. Clin Plast Surg 2011;38:229-39. [DOI] [PubMed] [Google Scholar]
- 3.Alonso-Burgos A, García-Tutor E, Bastarrika G, et al. Preoperative planning of deep inferior epigastric artery perforator flap reconstruction with multislice-CT angiography: imaging findings and initial experience. J Plast Reconstr Aesthet Surg 2006;59:585-93. [DOI] [PubMed] [Google Scholar]
- 4.Cina A, Barone-Adesi L, Rinaldi P, et al. Planning deep inferior epigastric perforator flaps for breast reconstruction: a comparison between multidetector computed tomography and magnetic resonance angiography. Eur Radiol 2013;23:2333-43. [DOI] [PubMed] [Google Scholar]
- 5.Masia J, Clavero JA, Larrañaga JR, et al. Multidetector-row computed tomography in the planning of abdominal perforator flaps. J Plast Reconstr Aesthet Surg 2006;59:594-9. [DOI] [PubMed] [Google Scholar]
- 6.Masia J, Kosutic D, Clavero JA, et al. Preoperative computed tomographic angiogram for deep inferior epigastric artery perforator flap breast reconstruction. J Reconstr Microsurg 2010;26:21-8. [DOI] [PubMed] [Google Scholar]
- 7.Pauchot J, Aubry S, Kastler A, et al. Preoperative imaging for deep inferior epigastric perforator flaps: a comparative study of computed tomographic angiography and magnetic resonance angiography. Eur J Plast Surg 2012;35:795-801. [Google Scholar]
- 8.Pellegrin A, Stocca T, Belgrano M, et al. Preoperative vascular mapping with multislice CT of deep inferior epigastric artery perforators in planning breast reconstruction after mastectomy. Radiol Med 2013;118:732-43. [DOI] [PubMed] [Google Scholar]
- 9.Rosson GD, Williams CG, Fishman EK, et al. 3D CT angiography of abdominal wall vascular perforators to plan DIEAP flaps. Microsurgery 2007;27:641-6. [DOI] [PubMed] [Google Scholar]
- 10.Rozen WM, Phillips TJ, Ashton MW, et al. Preoperative imaging for DIEA perforator flaps: a comparative study of computed tomographic angiography and Doppler ultrasound. Plast Reconstr Surg 2008;121:9-16. [DOI] [PubMed] [Google Scholar]
- 11.Scott JR, Liu D, Said H, et al. Computed tomographic angiography in planning abdomen-based microsurgical breast reconstruction: a comparison with color duplex ultrasound. Plast Reconstr Surg 2010;125:446-53. [DOI] [PubMed] [Google Scholar]
- 12.Tong WM, Dixon R, Ekis H, et al. The impact of preoperative CT angiography on breast reconstruction with abdominal perforator flaps. Ann Plast Surg 2012;68:525-30. [DOI] [PubMed] [Google Scholar]
- 13.Casey WJ, 3rd, Chew RT, Rebecca AM, et al. Advantages of preoperative computed tomography in deep inferior epigastric artery perforator flap breast reconstruction. Plast Reconstr Surg 2009;123:1148-55. [DOI] [PubMed] [Google Scholar]
- 14.Fansa H, Schirmer S, Frerichs O, et al. Significance of abdominal wall CT-angiography in planning DIEA perforator flaps, TRAM flaps and SIEA flaps. Handchir Mikrochir Plast Chir 2011;43:81-7. [DOI] [PubMed] [Google Scholar]
- 15.Gacto-Sánchez P, Sicilia-Castro D, Gómez-Cía T, et al. Computed tomographic angiography with VirSSPA three-dimensional software for perforator navigation improves perioperative outcomes in DIEP flap breast reconstruction. Plast Reconstr Surg 2010;125:24-31. [DOI] [PubMed] [Google Scholar]
- 16.Ghattaura A, Henton J, Jallali N, et al. One hundred cases of abdominal-based free flaps in breast reconstruction. The impact of preoperative computed tomographic angiography. J Plast Reconstr Aesthet Surg 2010;63:1597-601. [DOI] [PubMed] [Google Scholar]
- 17.Malhotra A, Chhaya N, Nsiah-Sarbeng P, et al. CT-guided deep inferior epigastric perforator (DIEP) flap localization -- better for the patient, the surgeon, and the hospital. Clin Radiol 2013;68:131-8. [DOI] [PubMed] [Google Scholar]
- 18.Minqiang X, Lanhua M, Jie L, et al. The value of multidetector-row CT angiography for pre-operative planning of breast reconstruction with deep inferior epigastric arterial perforator flaps. Br J Radiol 2010;83:40-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rozen WM, Anavekar NS, Ashton MW, et al. Does the preoperative imaging of perforators with CT angiography improve operative outcomes in breast reconstruction? Microsurgery 2008;28:516-23. [DOI] [PubMed] [Google Scholar]
- 20.Rozen WM, Ashton MW, Grinsell D, et al. Establishing the case for CT angiography in the preoperative imaging of abdominal wall perforators. Microsurgery 2008;28:306-13. [DOI] [PubMed] [Google Scholar]
- 21.Rozen WM, Ashton MW, Stella DL, et al. The accuracy of computed tomographic angiography for mapping the perforators of the DIEA: a cadaveric study. Plast Reconstr Surg 2008;122:363-9. [DOI] [PubMed] [Google Scholar]
- 22.Schaverien MV, Ludman CN, Neil-Dwyer J, et al. Contrast-enhanced magnetic resonance angiography for preoperative imaging of deep inferior epigastric artery perforator flaps: advantages and disadvantages compared with computed tomography angiography: a United Kingdom perspective. Ann Plast Surg 2011;67:671-4. [DOI] [PubMed] [Google Scholar]
- 23.Smit JM, Dimopoulou A, Liss AG, et al. Preoperative CT angiography reduces surgery time in perforator flap reconstruction. J Plast Reconstr Aesthet Surg 2009;62:1112-7. [DOI] [PubMed] [Google Scholar]
- 24.Uppal RS, Casaer B, Van Landuyt K, et al. The efficacy of preoperative mapping of perforators in reducing operative times and complications in perforator flap breast reconstruction. J Plast Reconstr Aesthet Surg 2009;62:859-64. [DOI] [PubMed] [Google Scholar]
- 25.Rozen WM, Phillips TJ, Stella DL, et al. Preoperative CT angiography for DIEP flaps: ‘must-have’ lessons for the radiologist. J Plast Reconstr Aesthet Surg 2009;62:e650-1. [DOI] [PubMed] [Google Scholar]
- 26.Pacifico MD, See MS, Cavale N, et al. Preoperative planning for DIEP breast reconstruction: early experience of the use of computerised tomography angiography with VoNavix 3D software for perforator navigation. J Plast Reconstr Aesthet Surg 2009;62:1464-9. [DOI] [PubMed] [Google Scholar]
- 27.Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging 2004;17:205-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rozen WM, Chubb D, Ashton MW, et al. Achieving high quality 3D computed tomographic angiography (CTA) images for preoperative perforator imaging: now easily accessible using freely available software. J Plast Reconstr Aesthet Surg 2011;64:e84-6. [DOI] [PubMed] [Google Scholar]
- 29.Moon HK, Taylor GI. The vascular anatomy of rectus abdominis musculocutaneous flaps based on the deep superior epigastric system. Plast Reconstr Surg 1988;82:815-32. [DOI] [PubMed] [Google Scholar]