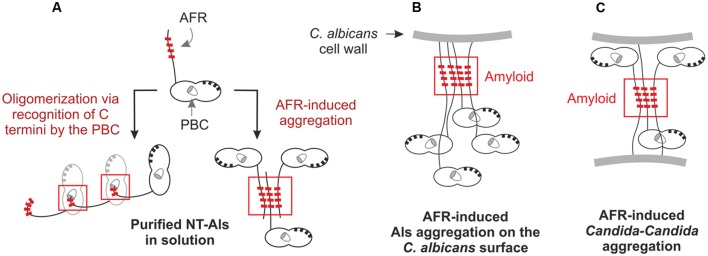
FIGURE 4.
Mechanisms of NT domain interactions between purified proteins (A) and between mature, full-length Als proteins on the C. albicans cell surface (B,C). (A) Purified NT-Als proteins may interact by two mechanisms. The first involves PBC-mediated recognition of the free C-terminal peptide, leading to oligomerization of the NT-Als molecules (left). The second mechanism involves aggregation mediated by the AFR (right). Because the NT domain is a small portion of the full-length, mature Als protein, PBC-mediated oligomerization of the proteins cannot explain aggregation between Als molecules on the C. albicans cell surface. These interactions are more likely attributable to the AFR (B). The AFR of mature, full-length Als proteins can also promote Als–Als-mediated aggregation between different C. albicans cells (C).