Abstract
Lifestyle choices influence 20–40 % of adult peak bone mass. Therefore, optimization of lifestyle factors known to influence peak bone mass and strength is an important strategy aimed at reducing risk of osteoporosis or low bone mass later in life. The National Osteoporosis Foundation has issued this scientific statement to provide evidence-based guidance and a national implementation strategy for the purpose of helping individuals achieve maximal peak bone mass early in life. In this scientific statement, we (1) report the results of an evidence-based review of the literature since 2000 on factors that influence achieving the full genetic potential for skeletal mass; (2) recommend lifestyle choices that promote maximal bone health throughout the lifespan; (3) outline a research agenda to address current gaps; and (4) identify implementation strategies. We conducted a systematic review of the role of individual nutrients, food patterns, special issues, contraceptives, and physical activity on bone mass and strength development in youth. An evidence grading system was applied to describe the strength of available evidence on these individual modifiable lifestyle factors that may (or may not) influence the development of peak bone mass (Table 1). A summary of the grades for each of these factors is given below. We describe the underpinning biology of these relationships as well as other factors for which a systematic review approach was not possible. Articles published since 2000, all of which followed the report by Heaney et al. [1] published in that year, were considered for this scientific statement. This current review is a systematic update of the previous review conducted by the National Osteoporosis Foundation [1].
| Lifestyle Factor | Grade |
| Macronutrients | |
| Fat | D |
| Protein | C |
| Micronutrients | |
| Calcium | A |
| Vitamin D | B |
| Micronutrients other than calcium and vitamin D | D |
| Food Patterns | |
| Dairy | B |
| Fiber | C |
| Fruits and vegetables | C |
| Detriment of cola and caffeinated beverages | C |
| Infant Nutrition | |
| Duration of breastfeeding | D |
| Breastfeeding versus formula feeding | D |
| Enriched formula feeding | D |
| Adolescent Special Issues | |
| Detriment of oral contraceptives | D |
| Detriment of DMPA injections | B |
| Detriment of alcohol | D |
| Detriment of smoking | C |
| Physical Activity and Exercise | |
| Effect on bone mass and density | A |
| Effect on bone structural outcomes | B |
Considering the evidence-based literature review, we recommend lifestyle choices that promote maximal bone health from childhood through young to late adolescence and outline a research agenda to address current gaps in knowledge. The best evidence (grade A) is available for positive effects of calcium intake and physical activity, especially during the late childhood and peripubertal years—a critical period for bone accretion. Good evidence is also available for a role of vitamin D and dairy consumption and a detriment of DMPA injections. However, more rigorous trial data on many other lifestyle choices are needed and this need is outlined in our research agenda. Implementation strategies for lifestyle modifications to promote development of peak bone mass and strength within one’s genetic potential require a multisectored (i.e., family, schools, healthcare systems) approach.
Keywords: Bone mineral content, Diet, Nutrition, Peak bone mass, Physical activity
Introduction
Bone accretion
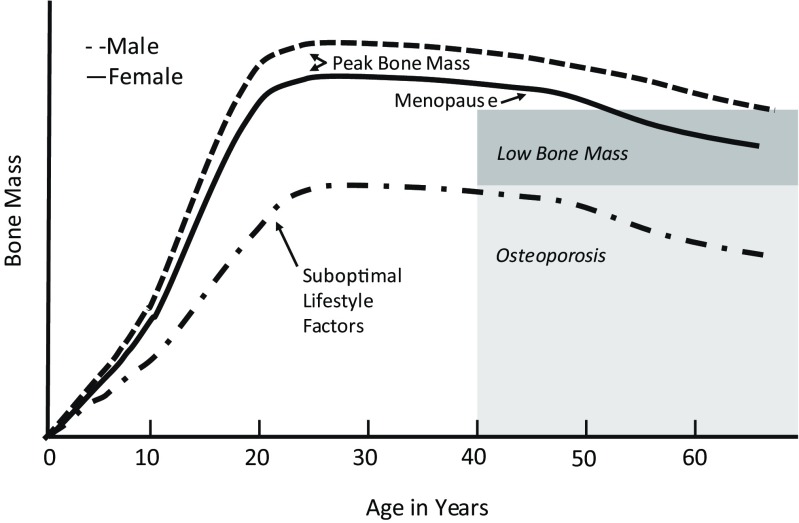
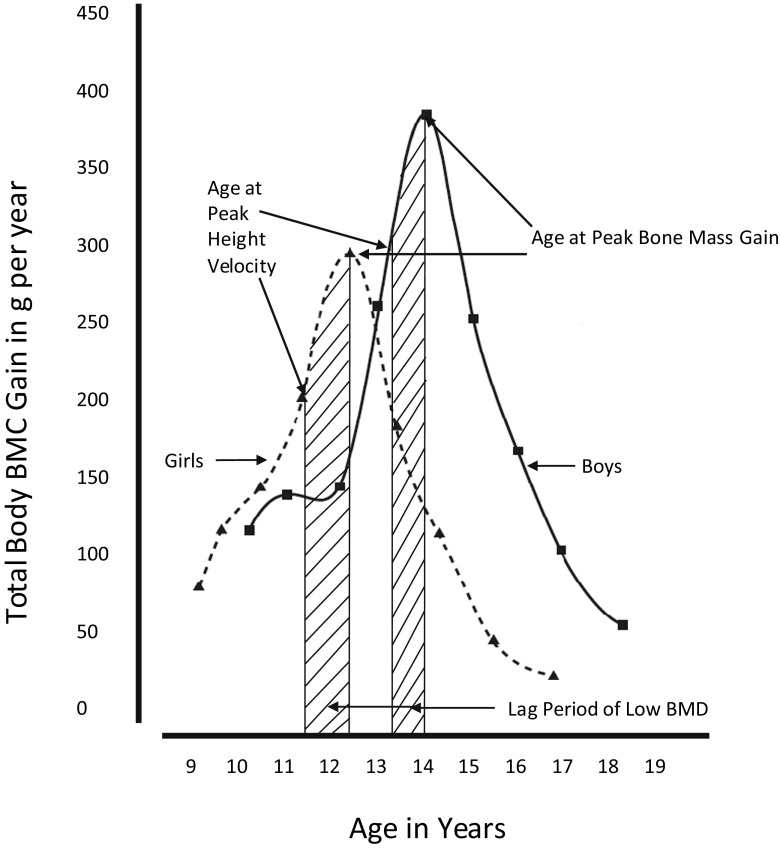
During growth and development, skeletal growth proceeds through the coordinated action of bone deposition and resorption to allow bones to expand (periosteal apposition of cortical bone) and lengthen (endochondral ossification) into their adult form [2]. This process of bone modeling begins during fetal growth and continues until epiphyseal fusion, usually by the end of the second decade of life [1]. Bone modeling is sensitive to mechanical loading, emphasizing the importance of physical activity throughout growth [2]. Some skeletal characteristics, such as cortical density and structural strength, determined by bone dimensions and thickness, continue to increase after epiphyseal fusion and into the third decade of life. Quantitatively, the amount of bone mineral acquired from birth to adulthood follows distinct age- and sex-specific patterns (Fig. 1). Bone mass is acquired relatively slowly throughout childhood. With the onset of puberty and the adolescent growth spurt in height, bone mineral accretion is rapid, reaching a peak shortly after peak height gain (Fig. 2). For total body bone mineral, the peak bone mineral accretion rate occurs at 12.5 ± 0.90 years in girls and 14.1 ± 0.95 years in boys of European ancestry [3]. During the 4 years surrounding the peak in bone accretion, 39 % of total body bone mineral is acquired; by 4 years following the peak, 95 % of adult bone mass has been achieved [4]. Within a population, the distribution of bone mass becomes more variable, in part due to differences in height and other skeletal dimensions as adult size is attained, the timing and magnitude of peak bone mineral accrual, the cessation of bone accretion, and lifestyle factors. This period of rapid accretion may be a time of both opportunity and vulnerability for optimizing peak bone mass.
Fig. 1.
Bone mass across the lifespan with optimal and suboptimal lifestyle choices
Fig. 2.
Peak BMC gain and peak height velocity in boys and girls from longitudinal DXA analysis. Adapted from Bailey et al. [3]
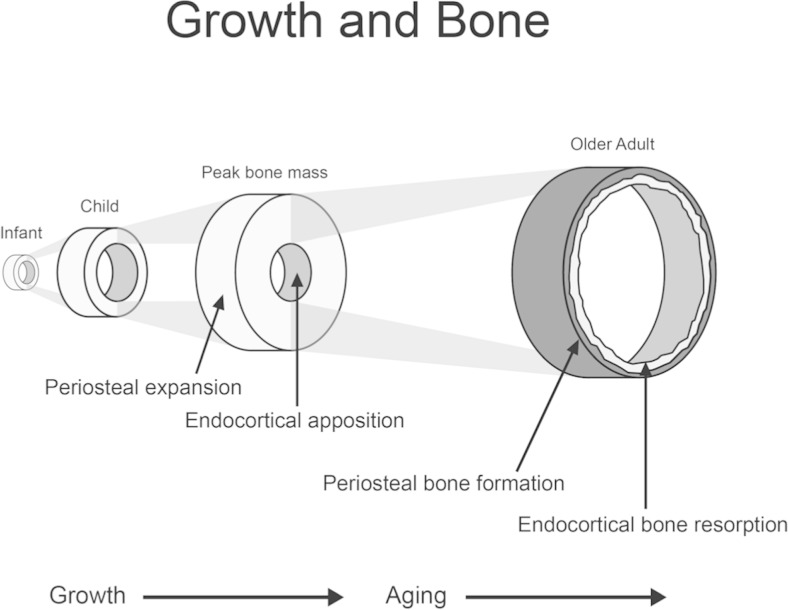
Changes in the structure (size and shape) and composition (amount of cartilage, cortical, and trabecular bone) of bone also occur with progression through puberty and thereby influence bone strength (Fig. 3). Cortical bone is the compact bone that forms the outer shell protecting bone marrow and trabecular bone. Trabecular bone is composed of rods and plates in a sponge-like structure, adding to the structural strength of bone. Cortical and trabecular bone differ in their responsiveness to disease effects, medications, muscle-loading and impact-loading physical activity, and hormonal changes. The relative importance of cortical versus trabecular bone in optimizing peak bone mass and strength and in minimizing fracture risk has not been firmly established in either childhood or adulthood. Distinct increases in trabecular bone of the spine and long bones occur between sexual maturity stages 3 and 4 [5–7]. The density of cortical bone is lower among children and adolescents than among adults, and it may even go through a transient period of increased porosity, particularly for boys [7, 8]. The density of cortical bone increases more rapidly as epiphyseal fusion occurs and continues into the third decade of life [9]. Both the inner and outer dimensions of long bones increase as growth proceeds, providing greater structural strength. The accumulation of bone mineral and changes in density and structural strength of bone may also continue into the third decade of life, depending on the bone compartment and skeletal site under consideration (Fig. 1).
Fig. 3.
Changes in structural composition of bone throughout the lifespan
Definition of peak bone mass
Peak bone mass is generally thought of as the amount of bone gained by the time a stable skeletal state has been attained during young adulthood. The concept of peak bone mass more broadly captures peak bone strength, which is characterized by mass, density, microarchitecture, microrepair mechanisms, and the geometric properties that provide structural strength.
There are several nuances to this concept that deserve recognition. The concept of peak bone mass is different when applied to an individual as opposed to a population. For an individual, peak bone mass may refer to the maximum amount of bone accrued during young adulthood. Alternatively, the concept of peak bone mass may refer to an individual’s maximal or genetic potential for bone strength (i.e., bone mineral content (BMC), areal bone mineral density (aBMD), or other measures of bone strength). At the population level, peak bone mass is attained when age-related changes in a bone outcome are no longer positive and have attained a plateau or maximum value [10].
Importance of peak bone mass
Fracture
Optimizing bone accrual during growth may be of greatest significance in preventing current or future fractures, as measures of bone mass, density, and structural strength are associated with fracture in children and adults [11–13]. The frequency of fractures is higher among children compared to young and middle-aged adults [14], reflecting the vulnerability of the growing skeleton prior to peak bone mass. Among healthy children, as many as one half of boys and one third of girls will sustain a fracture by age 18 years, with one fifth sustaining two or more fractures [15, 16]. Children who sustain a fracture before age 4 years are especially vulnerable to a subsequent fracture [17]. Thirty to 50 % of childhood fractures involve the forearm [14, 15, 18–20] and result from falls to an outstretched arm. There is a positive relationship between fracture frequency and level of physical activity due to the increased risk of falls during physical activity [21]. Thus, although physical activity is critical for bone modeling, children with higher levels of physical activity are more likely to have fractures [3, 22–28].
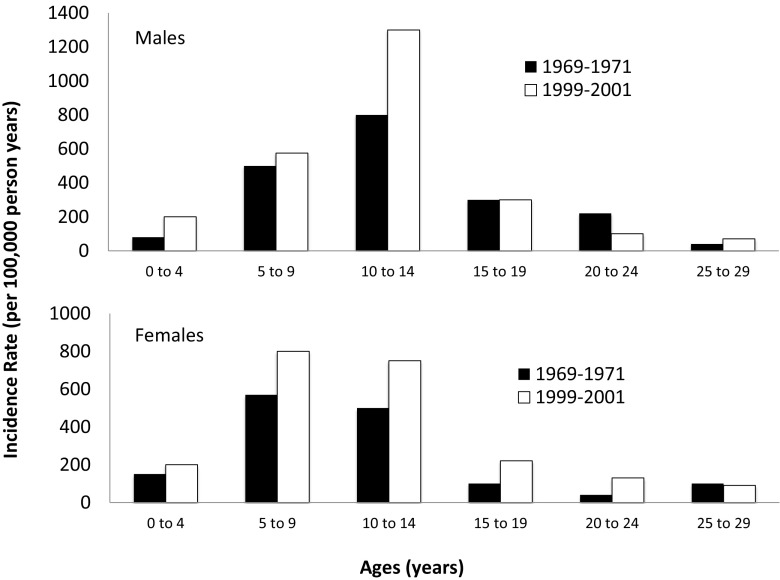
There is a developmental period during the rapid growth of late childhood and early adolescence when the skeleton is particularly vulnerable to fracture (Fig. 4) [29]. Recently, high-resolution peripheral quantitative computed tomography (HRpQCT) has been used to explain the microarchitectural basis for the observation of increased fracture frequency among young adolescents [7]. The combination of thinner cortical bone, lower total volumetric bone mineral density (vBMD), and increased cortical porosity, particularly in boys, suggests that linear bone growth outpaces bone mineralization, resulting in transient bone fragility.
Fig. 4.
Incidence of fractures of the distal forearm from birth through young adulthood. Adapted from Khosla et al. [29]
Understanding factors that affect bone strength early in life is important because low bone strength is associated with fracture risk in later life, independent of fall incidence and physical activity [30]. Childhood bone mass is predictive of fracture risk during childhood, with an 89 % increase in fracture risk per SD decrease in size-adjusted bone mass [31]. Moreover, among children who experience similar forearm injuries, those with greater bone density have been shown to be less likely to fracture [32]. Preterm children have low bone mass during late childhood [33], and birth weight is related to bone mass in later adult life (age ≥60 years) [34].
Recent work using HRpQCT suggests that microarchitectural changes underlie increased bone fragility in children who sustain a distal forearm fracture following mild trauma compared to nonfracture controls [35]. Differences such as cortical thinning are seen at both the distal radius and distal tibia in children presenting with a forearm fracture in which the degree of trauma is mild (e.g., fall from standing height), but not in those where the trauma is moderate (e.g., fall while riding a bicycle). Further analysis, including microfinite element analysis of HRpQCT data, showed that the mild trauma distal forearm fracture cases had reduced bone strength (i.e., failure load) compared to children without a fracture history. Moderate trauma is sufficient to break healthy bones that are not otherwise inherently at increased risk of fracture. Clark et al. [21] have shown that, irrespective of bone mass, fracture risk rises as the amount of vigorous activity increases. Additional studies have shown that a forearm fracture in a child is associated with lower areal and vBMD, cortical area, and bone strength using peripheral quantitative computed tomography (pQCT) and dual-energy x-ray absorptiometry (DXA) [11]. Cohort studies in the USA and South Africa show that boys and girls of European descent have a greater fracture risk than children of African descent [36, 37], a finding that parallels patterns of osteoporosis and hip fracture in elderly adults [38, 39].
In childhood and adolescence, stress fractures exhibit a different pattern from typical long bone fractures. The lifetime prevalence of stress fracture among the general population is below 4 % [40], and stress fractures are more common among women than among men [41]. In studies of military populations, where stress fractures are most common, the rate ratio may be 10:1 [42–47], with up to 20 % of female recruits in basic training reported to have sustained a stress fracture [44–48] (note: military studies include young adults aged ≥18 years). Risk factors for stress fractures among recruits include low quantitative ultrasound values, smoking, history of being sedentary [49], and volume of training [40, 44, 50]. White race and a reported family history of osteoporosis or osteopenia may also represent significant risk factors [51, 52].
Tracking
Tracking refers to the stability of a trait over time. The degree to which indicators of bone strength track from childhood to peak bone mass and beyond is of paramount importance to optimizing peak bone mass for lifelong skeletal health. If bone “status” (i.e., bone mass, density, or structural strength relative to one’s peers of the same age and sex) at any given time point were not associated with its future status, then concerns would only be relevant to prevention of childhood fractures, not osteoporosis later in life. In fact, numerous prospective studies have demonstrated that measures of bone density track quite strongly from childhood through adolescence, with tracking correlations ranging from 0.5 to 0.9 depending on the skeletal site, trait, and duration of follow-up, with most estimates falling in the range of 0.6 to 0.7 [32, 53–57]. Tracking correlations decline during adolescence and then rebound, a phenomenon that is likely due to variability in the timing of puberty and peak bone accrual. Adjustment for height status largely eliminates this transient decline in tracking [32, 57]. One study of children aged 8–16 years (n = 183) examined the factors associated with tracking deviation. Positive deviation (i.e., improvement in spine and hip aBMD tertile) was associated with having been breast-fed, gains in lean mass, aerobic fitness, and sports participation. Gains in adiposity were associated with negative deviations in tracking [55]. These findings provide strong evidence that bone status during childhood, when peak bone mass is accumulated, is indicative of bone status in young adulthood. However, the fact that tracking correlations are far from unity suggests that lifestyle factors can alter bone status in both positive and negative directions.
Timing of peak bone mass
If the magnitude of peak bone mass attained in young adulthood is an important predictor of osteoporosis later in life, then the timing of peak bone mass is also important because it defines the lifecycle phase during which peak bone mass can be optimized. Regardless of whether one is referring to peak bone mass of an individual or a population, the timing of peak bone mass varies by skeletal site. Estimates based on longitudinal studies are preferred over cross-sectional population studies for identifying the timing of peak bone mass because they capture the process of bone accretion. For example, using longitudinal observations and the plateau method, the Canadian Multicentre Osteoporosis Study identified the ages of peak bone mass for women; for lumbar spine aBMD, it was between the ages of 33 and 40 years, whereas ages of peak bone mass for total hip BMD were between 16 and 19 years [10].
Estimates of the timing of peak bone mass further depend on the parameters of bone (i.e., mass, density, geometry, microarchitecture) under consideration. Using quantitative computed tomography (QCT), Riggs et al. [9] showed that women aged 20–29 years (n = 15) were losing trabecular bone at a rate of 1–1.75 % per year at the distal radius and lumbar spine, but they were gaining cortical bone at a rate of 0.25 % per year in the tibia. By contrast, men (n = 8) in this age range did not exhibit significant changes in these outcomes [9]. Cross-sectional data on >1000 men, aged 18.0–20.9 years, in the Gothenburg Osteoporosis and Obesity Determinants Study suggest that aBMD of the lumbar spine, femoral neck, and total body did not increase with age, but positive age-related associations were observed for aBMD of the radius, cortical, and trabecular vBMD, and cortical thickness of the radius and tibia as measured by DXA and pQCT [58]. The positive association with cortical thickness was attributed to a smaller medullary diameter, and not to periosteal expansion.
Because the timing of peak bone mass and strength varies by skeletal site and bone compartment, it is important to establish and retain behaviors that contribute to skeletal health, including region-specific changes (e.g., hip, spine). Moreover, until the lifelong importance of peak bone mass is fully understood [52], it is prudent to assume that these behaviors are needed to sustain skeletal health through the life cycle.
Methods for measuring peak bone mass
Insights into the development of peak bone mass are based on studies using DXA and QCT. These measurement techniques characterize different aspects of bone strength; DXA primarily measures bone mass (or bone mineral content [BMC]) and aBMD, which are integrated measures of cortical and trabecular bone.QCT can provide distinct measures of cortical and trabecular vBMD, bone geometry (e.g., periosteal and endosteal circumference and structural strength) and, in some cases, microarchitecture.
Dual-energy x-ray absorptiometry
The vast majority of studies on peak bone mass have utilized DXA, a low-dose x-ray technology that measures the attenuation of x-ray beams as they pass through tissues of varying density. DXA is a two-dimensional imaging technique that uses a planar image to estimate bone area. This technology is ideal for use in children because it is rapid, safe, widely available, and precise, with effective dose ranges from 0.03 to 15.2 μSV [59]. Because of the smaller bone size and lower density of bones in growing children, special software has been developed by the major DXA manufacturers to measure aBMD and BMC in children. DXA does not measure vBMD but instead provides what is referred to as aBMD. Since DXA does not capture the depth of bone, it systematically underestimates vBMD in children with poor growth. For this reason, adjusting DXA measures of BMC and aBMD for stature is recommended [60, 61]. This adjustment serves to distinguish between gains in BMC or aBMD that are independent of gains in stature. In addition, cortical and trabecular bone are superimposed in the DXA image, thus providing a composite estimate of the mass and density of these two bone compartments.
Lack of agreement exists regarding whether BMC or aBMD should be the outcome of interest in bone accretion studies in children. BMC is determined, in large part, by bone size because it reflects the mineral content of one region or the entire skeleton; aBMD only partly adjusts for bone size and a size-related artifact remains [61]. Using spine QCT measures as a reference method, Wren and colleagues have shown that DXA BMC was a better measure to use in children (ages 6–17 years), particularly in prepubertal children, than aBMD [62]. We agree with those who argue that, to account for size in studies of children, it is best to use BMC adjusted for bone area [63, 64], height-for-age Z-score [61], lean mass [65, 66], or other combinations of anthropometric variables [64, 67, 68] or to use calculated bone mineral apparent density [69], because these provide a more accurate reflection of a child’s bone health.
DXA measures have also been used to estimate structural strength of the proximal femur using the hip structural analysis (HSA) algorithm [70]. HSA estimates subperiosteal width, cross-sectional area (CSA), and section modulus in the narrow neck, intertrochanteric region, and shaft of the proximal femur. These outcomes are associated with treatment effects in adults as well as disease and exercise effects in children and adolescents [71–73].
Peripheral quantitative computed tomography
DXA only partly describes bone strength, which is the broader concern for understanding peak bone mass. Other modalities are used to more directly measure vBMD, microarchitecture, and geometry. Many of these characteristics can easily be measured in children with relatively low radiation exposure (0.59–1.09 mSv) [74]. QCT and pQCT are three-dimensional techniques that also use attenuation of x-ray beams to construct bone images. Cortical and trabecular bone compartments vary in density, and the differential attenuation of x-ray beams in the three-dimensional reconstruction allows for separate determination of trabecular and cortical vBMD, as well as numerous other measures of bone geometry (e.g., total bone area, periosteal and endosteal circumference) and structural strength in compression, bending, and torsion (e.g., section modulus, strain–strength index). Full-sized computed tomography (CT) scanners are used to measure the spine and other sites, and dedicated pQCT scanners measure the radius, tibia, or distal femur. Newer HRpQCT scanners achieve sufficient resolution for building microstructural finite element models of whole bone failure load, a surrogate measure of bone’s resistance to fracture, as well as cortical porosity, and trabecular plate and rod microstructure [74].
Mechanical loading
Physical activity comprises any body movement produced by muscle contraction resulting in energy expenditure above a resting level [75]. Exercise is a more restrictive concept and is defined by planned, organized, and repetitive physical activity aimed at maintaining or enhancing one or more components of physical fitness or a specific health outcome, such as bone strength [68]. The randomized controlled trials (RCTs) reviewed in this scientific statement used targeted exercise as an intervention to improve bone strength, whereas most of the longitudinal studies measured physical activity, including active transportation and activities of everyday life [76]. Physical activity has long been regarded as behavior likely to influence bone health [77, 78]. Epidemiological and clinical trial research dating back more than two decades confirms the positive impact of regular physical activity on bone [3, 27, 78–81]. However, we are only beginning to quantify the specific dimensions, dose, and timing of physical activity needed for maximal bone strength. What is known, primarily from animal studies, is that increased mechanical loads placed on bone through both impact and muscle forces cause deformation (strains) of whole bone [82, 83]. These strains activate mechanosensitive cells (i.e., osteocytes), embedded within the bone, which signal molecules to activate osteoblasts and osteoclasts. The signaling begins the process of bone adaptation to changes in physical activity, as well as other mechanical loads (e.g., an increase in body weight). To initiate an osteogenic response, bone must be subjected to a strain magnitude that surpasses a threshold determined by the habitual strain range in the predominant loading direction. The threshold varies between individuals (and also bone sites) according to physical activity habits and other factors (e.g., maturity status). Thus, children and adolescents may respond differently to similar mechanical loading conditions. Inactive children may respond to low-impact loading and improve bone mass or structure, while more active children will need a higher mechanical load to promote a skeletal response [84].
The skeleton needs to be strong for load bearing and light for mobility. A manner of minimizing the amount of bone mass needed in a cross-section without decreasing strength is to modify the distribution of bone mass and therefore changing bone structure. Throughout life, but mainly during growth, periosteal apposition increases the diameter of long bones and endocortical resorption enlarges the marrow cavity. Cortical thickness is determined by the net changes occurring at the periosteal and endosteal surface of bone. However, even without an increase in cortical thickness, the displacement of the cortex increases bending strength because resistance to bending is proportional to the fourth power of the distance from the neutral axis. In addition to the independent effect of physical activity on mass and density, increased mechanical loading via physical activity may influence structural changes in bone to increase strength in response to the new loading condition [25, 73, 85].
Bone is most responsive to physical activities that are dynamic, moderate to high in load magnitude, short in load duration, odd or nonrepetitive in load direction, and applied quickly [84]. The load magnitude is produced by impact with the ground (e.g., tumbling or jumping), impact with an object (racquet sports), or muscle power moves such as the lift phase in jumping and vaulting. On the other hand, due to desensitization of the osteocytes, static loads and repetitive low-magnitude loads are not osteogenic [86–88]. Although physical activity is a modifiable factor that contributes to peak bone mass and strength, our understanding of how to quantify the dimensions of physical activity that are osteogenic (including frequency, intensity, time, and type) is incomplete.
Body composition
It is widely recognized that lean body mass is among the strongest correlates of bone mass, density, and structural strength during childhood [89–92]. During adolescence, the peak in total body lean mass accretion occurs just prior to peak bone mineral accretion [2, 93], although at specific sites, peak increases in lean mass and bone strength may be coordinated [94]. In the latter phase of the adolescent growth spurt, following the peak, continued gains in lean mass are strong predictors of increases in BMC [95].
A major challenge in understanding the relationship between lean mass and bone is that both lean mass and bone mass have a strong heritable component. A study of young adult twins (aged 23–31 years) found that additive genetic factors accounted for 87 % of the variation in total body BMD, 81 % of the variation in lean mass, and 69–88 % of the covariance between lean mass and BMD depending on the skeletal site. Population differences also provide evidence of genetic determinants of lean and bone mass. Cardel et al. [96] compared groups of African or European ancestry (n = 301, aged 7–12 years) using ancestry informative DNA markers and found that a greater amount of African admixture was associated with greater lean mass and BMC after adjusting for socioeconomic status, sex, age, height, race/ethnicity, and pubertal status.
The effect of fat mass on bone mineral accretion and attainment of peak bone mass is far more controversial. Generally, greater body weight increases the effects of weight-bearing activity on bone. As children grow and increase in weight, both lean and fat mass increase. To reduce the likelihood of confounding from the bone loading effects of lean mass, it is important to first account for the effect of lean mass on bone in order to determine the effects of fat mass.
The source of adipose tissue may be important in considering the effects of body composition on bone outcomes. Visceral adipose tissue has different metabolic effects compared to subcutaneous fat, and it may be deleterious to bone by reducing bone quality. Adipose infiltrations of muscle and bone marrow associated with excess adiposity also have adverse effects on bone. Muscle density measured by pQCT is lower when the fat content within muscle is increased.
Nonmodifiable factors
Genetics
An estimated 60–80 % of the variability in bone mass and osteoporosis risk is explained by heritable factors. aBMD is lower among daughters of women with osteoporosis [97] and in men and women with first-degree relatives who have osteoporosis [98]. The familial resemblance of BMC is expressed prior to puberty [99, 100]. Genome-wide association studies have identified more than 70 loci associated with adult bone density or fractures [101, 102]. However, only a few such studies have been conducted in children [1, 103–106]. Twin studies also suggest that genetic predisposition determines up to 80 % of peak bone mass; the remaining 20 % is modulated by environmental factors and sex hormone levels during puberty [107].
Population ancestry
In North America, ethnic differences in vBMD and aBMD have been reported in children [5, 108, 109]. Among individuals aged 9–25 years, aBMD was consistently greater at all sites for African Americans compared to other groups, whereas Caucasians had greater values than Asians and Hispanics. In studies comparing children of Asian, European, and Hispanic ancestry, group differences in BMC were attributable to differences in bone size [110–112]. Ethnic differences in the rate of BMD gain have also been observed [109]. Differences between Caucasians, Asians, and Hispanics are smaller than between blacks and other groups; thus, pediatric reference ranges for BMC and aBMD are presented for African Americans and non-African Americans, and the International Society for Clinical Densitometry recommends using race-specific reference ranges in childhood because they reflect genetic potential for bone accretion [60, 111]. Studies using QCT provide insights into the population ancestry differences in DXA measures by describing cortical bone dimensions and trabecular density [5, 113–115]. As noted earlier, trabecular density increases during puberty. The magnitude of the pubertal increase in trabecular density is greater in African-American individuals than in Caucasians, and African-American children have greater total femoral bone in cross-sectional analyses [5, 6, 115].
Sex
Among children and adolescents, males have greater BMC and aBMD than females. These differences become more pronounced with the onset and progression through puberty or at the ages that correspond to these maturational changes [108, 109, 116–118]. The exact age at which these differences emerge is unclear. Earlier studies of infants (aged ≤12 months) did not find sex differences in total body BMD [119, 120] or spine BMC and aBMD [121, 122]; however, males (aged 1–18 months) had greater total body BMC than females [123]. A recent study of infants and toddlers aged 1–36 months confirmed the absence of sex differences in aBMD in very young children but found greater BMC in males than in females. Sex differences in the body size of infants and toddlers may account for BMC differences and the absence of aBMD differences. By about 5 years of age, girls have lower values for spine and hip aBMD than boys, a finding that persists when adjusted for age, height, and weight [124].
Studies of bone strength by pQCT reveal a more complex pattern of sex differences. In a study of 665 healthy individuals aged 5–35 years, cortical BMC, periosteal circumference, and section modulus were lower in the 38 % site of the tibia for females compared with males across all stages of puberty. However, cortical vBMD was greater and endosteal circumference was lower in peripubertal and postpubertal females compared to males. These differences were not attributable to differences in muscle mass or bone size [115]. In a 20-month longitudinal study of 128 children across puberty, boys exhibited a 10 % greater increase in total area and cortical area compared to girls, but the increase in the size of the marrow cavity was significantly less for girls than for boys [125]. Further evaluation showed that sex differences in bone strength are primarily due to the 4–6 % greater bone area in boys, which is evident in prepubertal children [126]. HRpQCT studies of the radius show that girls have higher cortical vBMD in midpuberty and postpuberty (9.4 and 7.4 %, respectively) and lower cortical porosity than boys (−118 and −56 %, respectively) [127].
Maturation
Advancement through puberty is associated with increases in BMC and aBMD, as well as cortical and trabecular vBMD. Moreover, several studies suggest that the timing of maturation may affect peak bone mass, particularly in girls. For example, Gilsanz et al. [128] showed that earlier age of pubertal onset was associated with greater DXA BMC and aBMD at skeletal maturity in both boys and girls, independent of prepubertal BMC and aBMD values and duration of puberty. Chevalley et al. [129] found that girls who attained menarche earlier had higher aBMD at multiple skeletal sites prior to, during, and after puberty. A Canadian longitudinal study (depicted in Fig. 2) found that girls who mature early had 3–4 % more total body BMC at age 20 years than girls who matured at an average age. However, maturational effects were only observed at the total body and not at other sites; no maturational timing effects were observed in males [130]. The absence of a maturation timing effect on aBMD and BMC of the lumbar spine, femoral neck, and total body was confirmed in a study of Swedish military recruits in which young men were followed until age 24 years. However, as with girls, later puberty in boys was associated with lower radius aBMD (−4.2 %, by DXA), as well as lower cortical (−0.7 %) and trabecular vBMD (−4.8 %, by pQCT) [131]. The long-term consequences of the effect of pubertal timing on peak bone mass remain to be determined.
Modifiable factors
Diet and physical activity are the primary modifiable factors associated with bone health, although other lifestyle and environmental factors may also be at play. Here, we review these factors and their contribution to peak bone mass.
Although we separately address the contribution of physical activity to peak bone mass and strength, we address nutrient interactions with physical activity and their effects on bone in the respective nutrient discussions. Several narrative and meta-analysis review articles were recently published that also address the strength of the evidence for physical activity and bone development [132–137].
Scientific statement aims
In this scientific statement, we (1) report the results of an evidence-based review of the literature since 2000 on factors that influence achieving the full genetic potential for skeletal mass, (2) recommend lifestyle choices that promote maximal bone health throughout the lifespan, (3) outline a research agenda to address current gaps, and (4) identify implementation strategies.
Methods
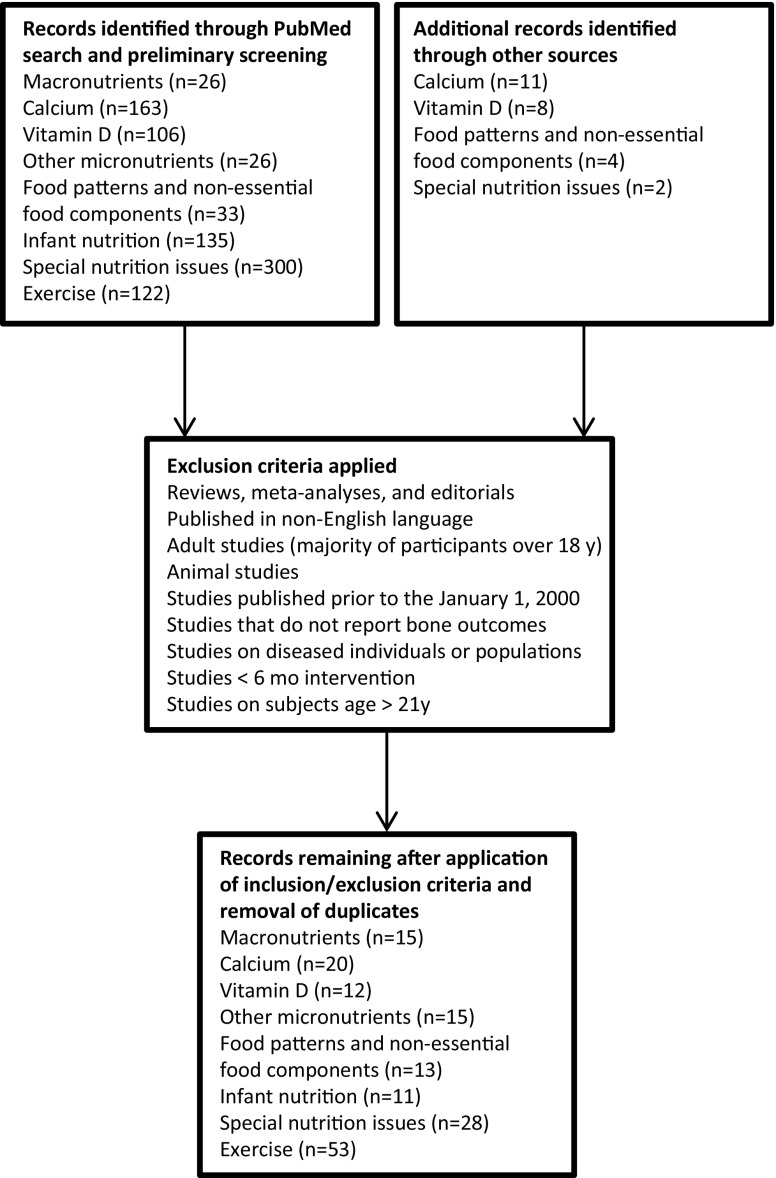
We performed a comprehensive PubMed (http://www.ncbi.nlm.nih.gov/pubmed) search of the scientific literature for articles published from January 2000 through December 2014. For all search terms, the following search strategy was used: ((((search term[Title/Abstract]) AND bone[Title/Abstract]) AND child*[Title/Abstract]) AND adolescen*[Title/Abstract]) NOT review[Publication Type]. Language, date, and species filters were then applied to the list of search results to eliminate articles not in English, articles published outside the 2000–2014 window, and animal studies. Searches for some of the topics required less restrictive searching in order to yield viable results, such as removal of the terms “child*” and/or “adolescen*,” or by expanding searches to scan terms found in “All Fields” rather than just “Title/Abstract.” MeSH terms were also utilized in some instances. Studies that contained subjects aged ≤21 years were included, except in the alcohol and smoking literature, in which studies that contained subjects aged ≤22 years were accepted due to lack of data in younger populations. Figure 5 represents the flow diagram of the systematic review for peak bone mass that includes search topics and the number of search returns.
Fig. 5.
Flow diagram of the systematic review on peak bone mass
To further narrow the search results for the broader topics (e.g., calcium, vitamin D, physical activity), we assigned authors to subcommittees based on their expertise and these subcommittees then reviewed the resultant abstracts. We excluded any articles that were not describing RCTs or observational studies, any studies that did not examine bone outcomes, and any interventions that were <6 months in duration. Studies and drug trials addressing disease states, with the exceptions of eating disorders and obesity, were likewise excluded. The articles that remained after the applications of these criteria were then rated based on the extent of scientific evidence as outlined in Table 1. This evidence grading system has previously been utilized by prominent organizations such as American Society for Nutrition [138] and the American Diabetes Association [139] and is recommended by other experts [140]. The assigned grade reflects the strength of available evidence on individual modifiable lifestyle factors that may (or may not) influence the development of peak bone mass. We assigned evidence grades after we achieved consensus among the writing group.
Table 1.
Evidence grading system
| Level of evidencea | Description |
|---|---|
| A: Strong | Clear evidence from at least one large, well-conducted, generalizable RCT that is adequately powered with a large effect size and is free of bias or other concerns |
| OR | |
| Clear evidence from multiple RCTs or many controlled trials that may have few limitations related to bias,measurement imprecision, inconsistent results, or other concerns | |
| B: Moderate | Evidence obtained from multiple, well-designed, conducted, and controlled prospective cohort studies that have used adequate and relevant measurements and that gave similar results from different populations |
| OR | |
| Evidence obtained from a well-conducted meta-analysis of prospective cohort studies from different populations | |
| C: Limited | Evidence obtained from multiple prospective cohort studies from diverse populations that have limitations related to bias, measurement imprecision, or inconsistent results or have other concerns |
| OR | |
| Evidence from only one well-designed prospective study with few limitations | |
| OR | |
| Evidence from multiple well-designed and conducted cross-sectional or case-controlled studies that have very few limitations that could invalidate the results from diverse populations | |
| OR | |
| Evidence from a meta-analysis that has design limitations | |
| D: Inadequate | Evidence from studies that have one or more major methodological flaws or many minor methodological flaws that result in low confidence in the effect estimate |
| OR | |
| Insufficient data to support a hypothesis | |
| OR | |
| Evidence derived from clinical experience, historical studies (before and after), or uncontrolled descriptive studies or case reports |
RCT randomized controlled trial
aRefers to the body of evidence
Tables 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, and 13
Table 2.
Fat and bone health in children and adolescents
| Macronutrient | Reference | Study description | Population description | Number of subjects | End points | Results | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prospective studies | |||||||||||
| Fat | Hogstrom et al. 2007 [141] | The objective of this study was to investigate the role of fatty acids in bone accumulation and the attainment of peak bone mass in young men | Sex: male Age: 16.7 years at baseline Race: white Location: Sweden Year(s): 1994 (baseline), first follow-up was at a mean of 6 years (age 22 years) |
78 | Data are shown for the overall group (N = 78) | Total body | Hip | Spine | |||
| r | P | r | P | r | P | ||||||
| Palmitic acid | −0.04 | NS | 01 | NS | 0.01 | NS | |||||
| Palmitoleic acid | 0.04 | NS | 0.03 | NS | 0.02 | NS | |||||
| Stearic acid | 0.07 | NS | 0.02 | NS | 0.04 | NS | |||||
| Oleic acid | −0.19 | NS | −0.10 | NS | −0.22 | NS | |||||
| Linoleic acid | 0.00 | NS | −0.06 | NS | −0.15 | NS | |||||
| Eicosatrienoic acid | −0.09 | NS | −0.04 | NS | −0.08 | NS | |||||
| Arachidonic acid | 0.12 | NS | 0.15 | NS | 0.25 | <0.05 | |||||
| Eicosapentaenoic acid | 0.04 | NS | 0.02 | NS | 0.16 | NS | |||||
| Docosapentaenoic acid | 0.02 | NS | −0.07 | NS | 0.05 | NS | |||||
| DHA | 0.10 | NS | 0.07 | NS | 0.26 | <0.05 | |||||
| PUFA | 0.14 | NS | 0.07 | NS | 0.16 | NS | |||||
| MUFA | −0.18 | NS | −0.09 | NS | −0.21 | NS | |||||
| SFA | 0.04 | NS | 0.03 | NS | 0.05 | NS | |||||
| n-6 | 0.04 | NS | 0.00 | NS | 0.07 | NS | |||||
| n-3 | 0.10 | NS | 0.07 | NS | 0.26 | <0.05 | |||||
| n-6:n-3 | −0.12 | NS | −0.13 | NS | −0.26 | <0.05 | |||||
| Data presented above include Pearson’s correlations between fatty acids measured in the phospholipid fraction for changes in aBMD from 16 to 22 years | |||||||||||
| Cross-sectional studies | |||||||||||
| Fat | Eriksson et al. 2009 [142] | Serum phospholipid fatty acid pattern was studied in relation to bone parameters in healthy children | Sex: male and female Mean age: 8.2 years Race: Caucasian Location: Gothenburg, Sweden Year(s): not specified |
85 | Data are shown for the overall group (N = 85) | Total body BMC (g) | |||||
| Correlation (r) | P | ||||||||||
| Palmitic acid (16:0) | 0.17 | NS | |||||||||
| Stearic acid (18:0) | −0.15 | NS | |||||||||
| Arachidic acid (20:0) | 0.20 | NS | |||||||||
| Nervonic acid (24:1n-9) | 0.27 | <0.05 | |||||||||
| Linoleic acid (18:2n-6) | −0.17 | NS | |||||||||
| Arachidonic acid (20:4n-6) | 0.23 | <0.05 | |||||||||
| α-Linolenic acid (18:3n-3) | −0.22 | <0.05 | |||||||||
| DHA (22:6n-3) | 0.03 | NS | |||||||||
| ∑ n-6 | −0.07 | NS | |||||||||
| ∑ n-3 | 0.03 | NS | |||||||||
| n-6:n-3 | −0.06 | NS | |||||||||
| Data presented above are from Pearson’s correlations | |||||||||||
25(OH)D 25-hydroxyvitamin D, aBMD areal bone mineral density, ANCOVA analysis of covariance, BMC bone mineral content, IGF insulin-like growth factor, NS not significant
Table 3.
Protein and bone health in children and adolescents
| Macronutrient | Reference | Study description | Population description | Number of subjects | End points | Results | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RCTs | |||||||||||
| Protein | Ballard et al. 2006 [143] | This study investigated whether 6 months of protein supplementation in conjunction with a strength and conditioning training program improves vBMD, bone geometry, and total body BMC. | Sex: male and female Age: 18–25 years Race: not specified Location: South Dakota, USA Year(s): not specified |
68 | Data are shown for the protein supplemented group (n = 36) | Mean change, protein group (n = 36) | P | ||||
| 4 % site | |||||||||||
| Total vBMD (mg/cm3) | 0.20 | NS | |||||||||
| Trabecular vBMD (mg/cm3) | −0.50 | NS | |||||||||
| Total area (cm2) | 5.0 | NS | |||||||||
| 20 % site | |||||||||||
| Cortical vBMD (mg/cm3) | 2.4 | NS | |||||||||
| Cortical area (cm2) | 1.7 | NS | |||||||||
| Cortical thickness (mm) | 0.05 | NS | |||||||||
| Periosteal circumference (mm) | −0.20 | NS | |||||||||
| Endosteal circumference (mm) | −0.50 | NS | |||||||||
| Polar SSI (mm3) | 57 | NS | |||||||||
| Total body | |||||||||||
| BMC (g) | −3.5 | NS | |||||||||
| Bone area (cm2) | −3.9 | NS | |||||||||
| Leg | |||||||||||
| BMC (g) | 1.3 | NS | |||||||||
| Arm | |||||||||||
| BMC (g) | 5.7 | NS | |||||||||
| Data presented above are least-squares means determined by ANCOVA while controlling for initial height and weight and baseline bone value. | |||||||||||
| Prospective studies | |||||||||||
| Protein | Alexy et al. 2005 [144] | This study examined whether the long-term dietary protein intake and diet net acid load are associated with bone status in children. In a prospective study design, long-term dietary intakes were calculated from 3-day weighed dietary records that were collected yearly over the 4-year period before a one-time bone analysis using pQCT. | Sex: male and female Age: 6–18 years Race: white Location: Dortmund, Germany Year(s): 1998–1999 subcohort of the DONALD study |
229 | Data are shown for the overall group (N = 229) | Protein (g/day) | |||||
| ß | ßstand | r 2 | P | ||||||||
| Forearm | |||||||||||
| Periosteal circumference (mm2) | 0.07 | 0.17 | 0.03 | <0.01 | |||||||
| Cortical area (mm2) | 0.42 | 0.27 | 0.04 | <0.01 | |||||||
| BMC (mg/mm) | 0.46 | 0.26 | 0.03 | <0.01 | |||||||
| Polar SSI (mm3) | 1.83 | 0.29 | 0.06 | <0.01 | |||||||
| • Data presented above are results from stepwise multiple regression and after adjustment for age, sex, and energy intake. • ßstand is the standardized parameter estimate. • Children with a higher dietary PRAL had significantly less cortical area (P < 0.05) and BMC (P < 0.01). • Long-term calcium intake had no significant effect on any bone variable. | |||||||||||
| Protein | Bounds et al. 2005 [146] | This study aimed to identify factors related to children’s bone mineral indexes at age 8 years, and to assess bone mineral indexes in the same children at ages 6 and 8 years. Children’s dietary intake and BMC were assessed as part of a longitudinal study from ages 2 months to 8 years. | Sex: male and female Age: 6 years (baseline) and 8 years (follow-up) Race: white Location: Knoxville, TN Year(s): not specified |
52 | Data are shown for the overall group (N = 229) | Protein intake (g) over ages 2–8 years | |||||
| r | P | ||||||||||
| Total body | |||||||||||
| BMC at age 8 years | 0.37 | ≤0.05 | |||||||||
| ß | Partial R 2 | P | |||||||||
| BMC model 1 | (+) 2.40 | 0.08 | <0.01 | ||||||||
| Data presented above show the Pearson’s correlation coefficient (r) relating protein intake over ages 2–8 years, representing 27 days of dietary data. BMC model 1 (R 2 = 0.69, F = 20.7, P < 0.01) | |||||||||||
| Protein | Vatanparast et al. 2007 [147] | This mixed-longitudinal study investigated the influence of protein intake on bone mass measures in young adults, considering the influence of calcium intake through adolescence. Dietary intake was assessed via serial 24-h recalls carried out at least once yearly. | Sex: male and female Age: 8–21 years during phase I of the study; 17–29 years for phase II Race: majority Caucasian Location: Saskatoon, Saskatchewan, Canada Year(s): 1991–1997 (phase I); 2003–2006 (phase II); participating in the University of Saskatchewan Pediatric Bone Mineral Accrual Study |
133 | Data are shown for the overall group (N = 133) and a subgroup (n = 44) | Protein intake (g) | |||||
| Regression coefficient | Partial R 2 | P | |||||||||
| Total body (N = 133) | |||||||||||
| BMC | NS | — | NS | ||||||||
| BMC net gain | 0.11 | 0.21 | 0.02 | ||||||||
| Total body (n = 44) | |||||||||||
| BMC | 0.21 | 0.33 | 0.04 | ||||||||
| BMC net gain | 0.21 | 0.37 | 0.02 | ||||||||
| The net gain of total body BMC and the net gains of height and weight from age of peak height velocity to early adulthood were entered into the model. Variables in the multiple regression model (stepwise) were sex, current height, weight, physical activity level, protein intake, vegetable and fruit intake, and periadolescence intakes of vegetables and fruit, protein, and physical activity. Protein intake predicted total body BMC net gain in all subjects. In females at periadolescence or early adulthood with adequate calcium intake(>1000 mg/day) (n = 44), protein intake positively predicted total body BMC and BMC net gain. | |||||||||||
| Protein | Zhang et al. 2010 [148] | This study assessed the association between protein intakes and bone mass accrual in girls who participated in a 5-year study including 2 years of milk supplementation (intervention groups only) and 3 years of follow-up study. | Sex: female Mean age: 10.1 years Race: Chinese Location: Beijing Year(s): 1999–2004 |
757 | Data are shown for the overall group (N = 757) | Average protein intake | |||||
| ß | P value | ||||||||||
| Total body | |||||||||||
| Bone area | – | NS | |||||||||
| BMC | −1.92 | 0.02 | |||||||||
| Proximal forearm | |||||||||||
| Bone area | −9.11 | <0.01 | |||||||||
| BMC | −10.2 | <0.01 | |||||||||
| Distal forearm | |||||||||||
| Bone area | – | NS | |||||||||
| BMC | −4.82 | <0.01 | |||||||||
| • Data presented above (ß) represent the percentage change in the dependent variable associated with intake of protein after controlling for baseline bone mass and pubertal development, age and physical activity, survey time, group, and clustering by schools. Protein, among other nutrients, was included in an initial model and flowed by backward elimination with P < 0.01 as the standard for retention, exclusion by the regression model. • When protein intake was considered according to animal or plant food sources, protein from animal foods, particularly meat, had significant negative effects on BMC accrual at the proximal and distal forearm (P < 0.05). | |||||||||||
| Protein | Remer et al. 2011 [145] | The aim of the study was to examine whether the association of long-term dietary acid load and protein intake with children’s bone status can be confirmed using approved urinary biomarkers and whether these diet influences may be independent of potential bone-anabolic sex steroids. Data were collected in 197 healthy children during the 4 years preceding proximal forearm bone analyses by pQCT. | Sex: male and female Age: 6–18 years Race: white Location: Dortmund, Germany Year(s): 1998–1999 subcohort of the DONALD study |
197 | Data are shown for the overall group (N = 197) | Urinary uN | Urinary PRAL | ||||
| ß | P | ß | P | ||||||||
| Forearm | |||||||||||
| BMC (mg/mm) [log 10] | 0.03 | <0.01 | −0.02 | 0.03 | |||||||
| Cortical area (mm2) [log 10] | 0.02 | <0.01 | −0.02 | 0.03 | |||||||
| Polar SSI (mm3) [log 10] | 0.02 | <0.01 | −0.01 | NS | |||||||
| Periosteal circumference (mm) | 0.50 | 0.03 | 0.02 | NS | |||||||
| BMD (mg/cm3) | 5.40 | NS | −8.70 | NS | |||||||
| • Data presented above are from multivariate regression models showing independent associations of both long-term protein intake (as uN) and PRAL as explanatory variables with forearm bone variables. Data are adjusted for age, sex, pubertal stage, forearm muscle area, forearm length, and urinary calcium. • Data show that 1 Z-score variation in uN leads to an average 7.2 % increase in BMC and a 4.7 % increase in cortical area as well as SSI. A 1 Z-score uN corresponds to 0.28-g protein intake/kg body wt, implying that an additional 1-g protein intake/kg body wt may lead to an average increase of 26 % in BMC and 17 % in cortical area and SSI. A 1-g protein intake/kg body wt is associated with an average increase of 1.8-mm periosteal circumference. | |||||||||||
| Cross-sectional studies | |||||||||||
| Protein | Hoppe et al. 2000 [149] | The objective of the study was to identify associations between dietary factors and total body bone measurements in a random sample of healthy Danish children. | Sex: male and female Age: 10 years Race: Danish, otherwise unspecified Location: Hvidovre, Denmark Year(s): 1997–1998, from the Copenhagen Cohort Study on Infant Nutrition and Growth |
105 | Data are shown for the overall group (N = 105) | Protein (g/day) | |||||
| Pearson’s r | P | ||||||||||
| Total body | |||||||||||
| Bone area (cm2) | 0.31 | <0.01 | |||||||||
| BMC (g) | 0.33 | <0.01 | |||||||||
| • The data above are unadjusted. • In the multiple linear regression analysis including height, weight, sex, energy intake, and bone-related nutrients in the model, dietary protein was not significantly associated with bone area or BMC. After backward elimination, in which height, weight, and sex were forced to stay in the model, dietary protein was positively associated with bone area (P < 0.05). • Inclusion of pubertal stages in the analyses did not alter the bone area or BMC outcomes. | |||||||||||
| Protein | Iuliano-Burns et al. 2005 [150] | This cross-sectional study assessed monozygotic and dizygotic male twin pairs to test the following hypotheses: (1) associations between bone mass and dimensions and exercise are greater than between bone mass and dimensions and protein or calcium intakes; and (2) exercise or nutrient intake are associated with appendicular bone mass before puberty and axial bone mass during and after puberty. | Sex: male Age: 7–20 years Race: not specified Location: Melbourne, Australia Year(s): 1997–2001 |
112 (56 twin pairs) | Data are shown for the overall group (N = 112) | Differences in protein | |||||
| Univariate | Size adjusted | All lifestyle and size adjusted | |||||||||
| ß | P | ß | P | ß | P | ||||||
| Differences in BMC (g) | |||||||||||
| Total body | 3.5 | NS | 1.3 | NS | 1.3 | NS | |||||
| Arms | 0.8 | <0.05 | 0.7 | <0.05 | 0.8 | <0.05 | |||||
| Legs | 1.6 | NS | 0.3 | NS | 0.3 | NS | |||||
| Lumbar spine | 0.0 | NS | 0.0 | NS | 0.0 | NS | |||||
| Differences in BMC (%) | |||||||||||
| Total body | 0.3 | <0.05 | 0.2 | <0.05 | 0.1 | NS | |||||
| Arms | 0.4 | <0.05 | 0.4 | <0.01 | 0.4 | <0.05 | |||||
| Legs | 0.3 | NS | 0.1 | NS | 0.1 | NS | |||||
| Lumbar spine | 0.1 | NS | 0.1 | NS | 0.2 | NS | |||||
| Differences in bone dimensions (mm) | |||||||||||
| Cortical thickness | 0.0 | NS | 0.0 | NS | 0.0 | NS | |||||
| Periosteal diameter | 0.0 | NS | 0.0 | NS | 0.0 | NS | |||||
| Endosteal diameter | 0.0 | NS | 0.0 | NS | −0.0 | NS | |||||
| Differences in bone dimensions (%) | |||||||||||
| Cortical thickness | 0.2 | NS | 0.1 | NS | 0.4 | NS | |||||
| Periosteal diameter | 0.1 | NS | 0.0 | NS | 0.0 | NS | |||||
| Endosteal diameter | −0.0 | NS | −0.1 | NS | −0.3 | NS | |||||
| • Data presented above are ß coefficients for within-pair differences in protein versus (1) within-pair differences in BMC and bone dimensions, (2) within-pair differences in size-adjusted BMC and bone dimensions, and (3) when all within-pair differences in protein, calcium, exercise duration, and size are included in the regression equation. • A 1-g difference in protein intake was associated with a 0.8-g (0.4 %) difference in arm BMC (P < 0.05). These relationships were present in peripubertal and postpubertal pairs but not in prepubertal pairs. Exercise during growth appears to have greater skeletal benefits than variations in protein or calcium intakes, with the site-specific effects evident in more mature twins. | |||||||||||
| Protein | Chevalley et al. 2008 [151] | This study analyzed the relationship between physical activity levels and protein compared with calcium intake on BMC in healthy prepubertal boys. | Sex: male Age: 6.5–8.5 years Race: white Location: Geneva, Switzerland Year(s): recruitment period 1999–2000, otherwise unspecified |
232 | Data are shown for the overall group (N = 232) | Correlation with protein intake (g/day) | |||||
| r | P | ßAdjusted | P | ||||||||
| BMC (g) | |||||||||||
| Radial metaphysis | 0.26 | <0.01 | 0.20 | 0.01 | |||||||
| Radial diaphysis | 0.21 | <0.01 | 0.12 | NS | |||||||
| Total radius | 0.27 | <0.01 | 0.20 | 0.01 | |||||||
| Femoral neck | 0.20 | <0.01 | 0.19 | 0.03 | |||||||
| Total hip | 0.18 | <0.01 | 0.12 | NS | |||||||
| Femoral diaphysis | 0.23 | <0.01 | 0.19 | 0.03 | |||||||
| Lumbar spine | 0.24 | <0.01 | 0.22 | <0.01 | |||||||
| Data presented above are from univariate (r) and multivariate (ßAdjusted) analyses; the latter takes into account the respective contribution of physical activity, protein, and calcium intakes. | |||||||||||
| Protein | Esterle et al. 2009 [152] | This study explored dietary calcium sources and nutrients possibly associated with lumbar bone mineralization and calcium metabolism in adolescent girls and evaluated the possible influence of a genetic polymorphic trait associated with adult-type hypolactasia. | Sex: female Age: 12–22 years Race: Caucasian Location: France Year(s): not specified |
192 | Data are shown for the postmenarchal group only (n = 142) | Protein from milk | Protein from other foods | ||||
| Adj R 2 | ßstand | P | Adj R 2 | ßstand | P | ||||||
| Lumbar spine BMC (g) | |||||||||||
| Absolute | 0.61 | 0.14 | <0.01 | 0.61 | <0.01 | NS | |||||
| Adjusted | 0.60 | 0.13 | 0.02 | 0.60 | <0.01 | NS | |||||
| • Data presented above are from multivariate linear analyses. • Absolute protein intake is expressed in g/days. • Adjusted protein intake for weight, years after menarche, and vertebral area is expressed in g/kg/days. • Girls with milk intakes <55 mL/day had significantly lower BMC compared to girls consuming >260 mL/day. • Neither BMC nor milk consumption was associated with −13,910 LCT polymorphism. | |||||||||||
| Protein | Ekbote et al. 2011 [153] | The aim of this study was to examine lifestyle factors as determinants of total body BMC and bone area in Indian preschool children. | Sex: male and female Age: 2–3 years Race: Indian Location: Pune, India Year(s): 2009 |
71 | Data are shown for the overall group (N = 71) | Protein (g/days) | |||||
| Normal | Malnourished | All | |||||||||
| r | P | r | P | r | P | ||||||
| Total body | |||||||||||
| Bone area | 0.65 | <0.01 | 0.57 | <0.01 | 0.58 | <0.05 | |||||
| BMC | 0.62 | <0.01 | 0.44 | <0.05 | 0.55 | <0.01 | |||||
| Data presented above are from Pearson’s correlation coefficients correlating protein intake and bone among normal children, malnourished children, and all (normal and malnourished children combined). | |||||||||||
| Protein | Libuda et al. 2011 [154] | This study examined relevant nutrients that are supposed to have an impact on bone parameters and compared their effect sizes with those of known predictors of bone development. | Sex: male and female Median age: 8.1 years Race: white Location: Dortmund, Germany Year(s): 1998–1999 subcohort of the DONALD study |
107 | Data are shown for the overall group (N = 107) | Protein (g/MJ) | |||||
| ß | ßstand | R 2 | P | ||||||||
| Forearm | |||||||||||
| Polar SSI (mm3) | – | – | – | NS | |||||||
| Periosteal circumference (mm) | – | – | – | NS | |||||||
| BMC (mg/mm) | 1.49 | 0.11 | 0.01 | NS | |||||||
| Cortical area (mm2) | 1.37 | 0.11 | 0.01 | NS | |||||||
| • Data presented above are from stepwise linear regression analyses, considering muscle area, BMI standard deviation scores, body fat %, age, sex, and rostenediol, as well as intakes of protein, calcium, vitamin D, and PRAL. • Of all nutrients considered, only protein showed a trend for an association with BMC (P = 0.073) and cortical area (P = 0.056) in stepwise linear regression models. • None of the other dietary variables were associated with bone parameters. • The protein effect did not differ between sexes. | |||||||||||
Adj adjusted, ANCOVA analysis of covariance, BMC bone mineral content, BMD bone mineral density, DONALD Dortmund Nutritional and Anthropometric Longitudinally Designed Study, NS not significant, pQCT peripheral quantitative computed tomography, PRAL potential renal acid load, RCT randomized controlled trial, SSI stress–strain index, uN urinary nitrogen, vBMD volumetric bone mineral density
Table 4.
Calcium and bone health in children and adolescents
| Nutrient | Reference | Study description | Population description | Number of subjects | End points | Results | ||
|---|---|---|---|---|---|---|---|---|
| RCTs | ||||||||
| Supplements | ||||||||
| Calcium carbonate, 1000 mg/day | Dibba et al. 2000 [160] | 12-month randomized, double-blind, placebo-controlled study Supplementation increased calcium intake from 342 to 1056 mg/day By young adulthood, there was no difference in the amount of bone accrued (mineral or size) or the rate of bone growth between supplemented and placebo. |
Sex: 80 boys, 80 girls Age: 8.3–11.9 year Race: Gambian, otherwise unspecified Location: rural Gambia, West Africa |
160 | Difference in % gain between groups | |||
| Midshaft arm BMC | 4.6 ± 0.9a | |||||||
| Distal radius BMC | 5.5 ± 2.7a | |||||||
| Adjusted for bone width, weight, and height | ||||||||
| Calcium carbonate, 800 mg + 400 IU vitamin 3/day | Moyer-Mileur et al. 2003 [163] | 1-year double-blind RCT Baseline calcium: not reported Average intake during study: supplement, 1524 (353); placebo, 906 (345); 30 % dropout Trabecular vBMD values were significantly greater in the supplemented group at baseline. |
Sex: female Age: 12 years; Tanner stage 2 Race: white Location: USA |
100 | Tibial pQCT measurements | Calcium and D | Placebo | |
| Trabecular vBMD % increasea | 1.0 | −2.0 | ||||||
| Trabecular BMC % increasea | 4.1 | 1.6 | ||||||
| Adjusted for baseline values | ||||||||
| Elemental calcium, 1000 mg/day | Rozen et al. 2003 [330]; Dodiuk-Gad et al. 2005 [331] | 12-month double-blind, placebo-controlled Habitual calcium intakes <800 mg/day Compliance dropped from 71 ± 26 % during the initial 6 months to 56 ± 34 % for the remaining study period (P = 0.0001) In follow-up study by Dodiuk-Gad et al., girls who have had compliance of ≥75 % on supplementation had significantly higher total body BMD after 3.5 years after supplementation than controls. |
Sex: female; >1 year postmenarchal Age: 12–17 years Race: 85 Jewish girls and 27 Arab girls Location: Haifa, Israel |
112 | Calcium supplement | Placebo | ||
| Lumbar spine BMC | 4.52 ± 0.48 | 3.95 ± 0.58 | ||||||
| Total body BMC | 4.63 ± 0.42 | 4.65 ± 0.54 | ||||||
| Femoral neck BMC | 4.30 ± 0.86 | 3.00 ± 0.81 | ||||||
| Lumbar spine BMD | 3.66 ± 0.35a | 3.00 ± 0.43 | ||||||
| Total body BMD | 3.80 ± 0.30a | 3.07 ± 0.29 | ||||||
| Femoral neck BMD | 2.00 ± 0.51 | 1.39 ± 0.42 | ||||||
| Not adjusted | ||||||||
| Calcium carbonate (Caltrate) supplement, 1200 mg/day | Cameron et al. 2004 [161] | 24-month RCT of twins Baseline calcium intake: 786, trt; 772, control in those who completed 24 months All were premenarchal at baseline 24 (38 %) of pairs completed 24 months Compliance was 76 % for both groups |
Sex: female Age: 8–13 years Race: not specified Location: Australia |
103 (50 twin pairs + 1 set of triplets) |
End of 24-month intention to treat | Difference in % gain between groups | ||
| Total body BMC | 3.69a | |||||||
| Hip BMD | −0.39a | |||||||
| Spine BMD | 1.40a | |||||||
| Femoral neck BMD | −0.57 | |||||||
| End of 12 months | ||||||||
| Total body BMC | 2.47a | |||||||
| Hip BMD | 1.64a | |||||||
| Spine BMD | 1.64a | |||||||
| Femoral neck BMD | 1.13 | |||||||
| Adjusted for age, height, and weight | ||||||||
| Calcium carbonate, 500 mg/day | Molgaard et al. 2004 [332] | 12-month randomized, double-blind, placebo-controlled intervention Subjects stratified at randomization according to baseline calcium intake Group A (n = 60) habitually consumed 1000–1307 mg/day (40th–60th percentile), and group B (n = 53) habitually consumed <713 mg/day (<20th percentile) |
Sex: female Age: 12 years ± 6 months Race: white Location: Denmark |
113 | No significant interaction between habitual calcium intake (groups A and B) and the intervention (Calcium carbonate–Placebo) in the analyses of height, weight, BMC, size-adjusted BMC, bone area, or BMD (all P > 0.15). When groups A and B were analyzed together, there was a significant effect of the intervention on BMD (0.8 %; 95 % CI, 0.01 %, 1.54 %; P = 0.049). |
|||
| Calcium citrate malate, 1000 mg/day | Matkovic et al. 2005 [333] | 4-year randomized clinical trial and optionally extended for an additional 3 years Baseline calcium: 830 ± 236 mg/day 51 % of the subjects completed the 7-year trial. |
Sex: female Age: 11 years; Tanner 2 Race: white Location: USA |
354 | Calcium supplement | Placebo | ||
| End of 4-year gain | ||||||||
| Total body BMD (g/cm2) | 0.215 ± 0.037a | 0.204 ± 0.0353 | ||||||
| Proximal radius BMD (g/cm2) | 0.167 ± 0.088 | 0.171 ± 0.079 | ||||||
| Distal radius BMD (g/cm2) | 0.106 ± 0.047a | 0.092 ± 0.046 | ||||||
| Cortical area/total area | 0.079 ± 0.021 | 0.072 ± 0.0213 | ||||||
| End of 7-year gain | ||||||||
| Total body BMD (g/cm2) | 0.268 ± 0.049 | 0.263 ± 0.044 | ||||||
| Proximal radius BMD (g/cm2) | 0.162 ± 0.038 | 0.156 ± 0.036 | ||||||
| Distal radius BMD (g/cm2) | 0.171 ± 0.047 | 0.165 ± 0.040 | ||||||
| Cortical area/total area | 0.095 ± 0.025a | 0.085 ± 0.0242 | ||||||
| By young adulthood, significant effects remained at metacarpals and at the forearm of tall persons. | ||||||||
| Calcichew, 1000 mg/day | Prentice et al. 2005 [162] | 13-month randomized, double-blind, placebo-controlled study Subjects were stratified by high or low exercise at baseline. Those in the low group were randomized to exercise intervention or none. Compliance with exercise was poor and no statistically significant differences were found between groups. For final analysis, all exercise intervention groups were combined. Subjects were told to not take the supplement with meals. Baseline calcium: 1197, supplement; 1199, placebo Total calcium intake during study: supplement, 1858 ± 629; placebo, 1283 ± 586 Latter factored in compliance |
Sex: male Age: 16–18 years Race: 90 % white; 10 % from various ethnic groups Location: Cambridge, UK |
143 | Difference in % gain between groups | |||
| Total body BMC | 0.33 ± 0.34 | |||||||
| Lumbar spine BMC | 0.18 ± 0.54 | |||||||
| Total hip BMC | 1.09 ± 0.54a | |||||||
| Femoral neck BMC | 1.21 ± 0.65 | |||||||
| Intertrochanter BMC | 0.99 ± 0.56 | |||||||
| Trochanter BMC | 0.29 ± 0.80 | |||||||
| Ultradistal radius BMC | 0.36 ± 0.86 | |||||||
| Adjusted for bone area, weight, and height | ||||||||
| Calcium carbonate, 800 mg/day and vitamin D3 400 IU/day | Greene and Naughton 2011 [164] | 6-month randomized placebo-controlled trial Baseline calcium: 763–786 mg/day |
Sex: female; identical twins Age: 9–13 years Race: not indicated Location: Australia |
40 (20 pairs) | pQCT | Tibial difference in % gain between groups | ||
| 4 % location | ||||||||
| Trabecular vBMD (mg/mm3) | 5.2 ± 1.96a | |||||||
| Trabecular area (mm2) | 5.4 ± 1.33a | |||||||
| Subcort density (mg/mm3) | 3.4 ± 0.82 | |||||||
| Subcortical area (mm2) | 0.8 ± 0.07 | |||||||
| SSI (mm3) | 6.6 ± 1.26a | |||||||
| 14 % | ||||||||
| Cortical BMD (mg/mm3) | 0.1 ± 0.04 | |||||||
| Cortical area (mm2) | 1.3 ± 0.35 | |||||||
| Total bone area (mm2) | 0.3 ± 0.02 | |||||||
| Medullary CSA (mm2) | −0.4 ± 0.02 | |||||||
| SSI (mm3) | 1.7 ± 0.22 | |||||||
| 38 % | ||||||||
| Cortical BMD (mg/mm3) | 0.8 ± 0.03 | |||||||
| Cortical area (mm2) | 5.8 ± 0.8a | |||||||
| Total bone area (mm2) | 0.5 ± 0.03 | |||||||
| Medullary CSA (mm2) | −6.2 ± 1.6a | |||||||
| SSI (mm3) | 0.7 ± 0.06 | |||||||
| 66 % | ||||||||
| Cortical BMD (mg/mm3) | 0.1 ± 0.02 | |||||||
| Cortical area (mm2) | 5.7 ± 0.39a | |||||||
| Total bone area (mm2) | −0.7 ± 0.03 | |||||||
| Medullary CSA (mm2) | −8.1 ± 1.82a | |||||||
| Muscle CSA (mm2) | 0.6 ± 0.02 | |||||||
| Radial difference in % gain between groups | ||||||||
| 4 % location | ||||||||
| Trabecular vBMD (mg/mm3) | 3.3 ± 0.28a | |||||||
| Trabecular area (mm2) | 2.8 ± 0.36a | |||||||
| Subcortical density (mg/mm3) | 0.1 ± 0.03 | |||||||
| Subcortical area (mm2) | 0.3 ± 0.01 | |||||||
| SSI (mm3) | 5.7 ± 0.51a | |||||||
| Calcium carbonate pills | Khadilkar et al. 2012 [334] | 1-year double-blind RCT of calcium, multivitamin with zinc, and vitamin D3 | Sex: female Age: 8–12 years Race: Indian Location: Pune, India |
214 | Total body BMC increase | Percent gain by group | ||
| Calcium + multivitamin | Calcium | Control | ||||||
| 21.5 ± 5.7a | 23.1 ± 6.1a | 19.4 ± 4.2 | ||||||
| Adjusted for Tanner stage and lean body mass | ||||||||
| Calcium, 1000 mg/day by pill or cheese Vitamin D, 800 IU/day |
Cheng et al. 2005 [159] | 2-year RCT; randomized to 1 of 4 groups: calcium 1000 mg/day + vitamin D 200 IU/day; calcium 1000 mg/day; cheese (1000 mg/day); placebo Baseline calcium 664–680 mg/day |
Sex: female Age: 10–12 years Race: not indicated Location: Finland |
195 | Calcium + vitamin D | Calcium | Placebo | |
| DXA | ||||||||
| Total body BMC (%) | 34.7 | 35.0 | 35.0 | |||||
| Femoral neck BMC (%) | 24.0 | 23.3 | 22.4 | |||||
| Total femur BMC (%) | 33.6 | 36.4 | 33.6 | |||||
| pQCT | ||||||||
| Radius CSA | 23.0 | 26.0 | 21.3 | |||||
| Radius BMC | 22.6 | 24.4 | 22.2 | |||||
| Tibial CSA | 15.6 | 15.8 | 14.8 | |||||
| Tibial BMC | 23.0 | 24.3 | 22.7 | |||||
| No significant differences | ||||||||
| Fortified foods | ||||||||
| Calcium citrate-malate, 792 mg/day dissolved in a fruit drink | Lambert et al. 2008 [165] | 18-month randomized trial with follow-up 2 years after supplement withdrawal Low calcium intake at baseline (mean 636 mg/day) Differences in gain no longer apparent 2 years after supplement withdrawal |
Sex: 96 girls Age: 11–12 years Race: white Location: Sheffield, UK |
96 | Calcium supplement | Placebo | ||
| Total body BMC (g) | 1698 ± 14a | 1667 ± 14 | ||||||
| Lumbar spine BMC | 43.0 ± 0.6a | 41.1 ± 0.6 | ||||||
| Total hip BMC | 27.7 ± 0.3 | 27.2 ± 0.4 | ||||||
| Adjusted for baseline values, age at menarche, and age at menarche × visit interaction | ||||||||
| Calcium-enriched beverage, 1200 mg/day | Gibbons et al. 2004 [166] | 18-month placebo-controlled, double-blind RCT of a high-calcium beverage. Controls received 400 mg/day Baseline calcium intake: 934, supplement; 985, placebo 12-month additional follow-up after end of intervention |
Sex: male and female Age: 8–10 years Race: NZ European or Pakeha Location: New Zealand |
154 | Treatment | Control | ||
| Total body BMD | 4.4 % | 3.3 % (NS) | ||||||
| Hip BMD | 4.8 % | 3.9 % (NS) | ||||||
| Spine BMD | 5.9 % | 5.8 % (NS) | ||||||
| Trochanter BMD | 6.2 % | 5.1 % (NS) | ||||||
| Femoral neck BMD | 6.7 % | 7.0 % (NS) | ||||||
| Calcium-fortified foods, 850 mg/day | Chevalley et al. 2005 [168] | 1-year double-blind RCT of calcium-fortified foods (850 mg/day) compared to an isocaloric control | Sex: male Age: 6.5–8.5 years Race: white Location: Switzerland |
235 | Spine BMD (L2–4) Femoral diaphysis BMD Femoral neck BMD Trochanter BMD Radius BMD |
No difference in gain 19 % greater increase in trt (P < 0.006) 3 % lesser increase (NS) 22 % greater (NS) 15 % greater (NS) |
||
| 600 mg calcium in 375 mL soymilk | Ho et al. 2005 [167] | 1-year follow-up study between 104 adolescent girls receiving the fortified food and 95 girls in the control group | Sex: female Age: 14–16 years Race: Chinese Location: Hong Kong, China |
210 | Mean % change treatment ± SD | Mean % change, control | ||
| Neck of the femur BMD | 2.7 ± 2.94 | 1.8 ± 3.49 | ||||||
| Trochanter BMD | 3.3 ± 3.27a | 1.6 ± 2.94 | ||||||
| Intertrochanter BMD | 3.6 ± 3.05a | 2.32 ± 2.95 | ||||||
| Total hip BMD | 3.1 ± 2.39a | 2.05 ± 2.22 | ||||||
| Total hip BMC | 3.8 ± 3.05a | 2.6 ± 2.96 | ||||||
| Dairy foods | ||||||||
| Dairy foods, 1000 mg/day | Merrilees et al. 2000 [169] | 2-year RCT of dairy foods Baseline calcium: 744, supplement; 765, control 1 year after end of supplementation, differences no longer seen |
Sex: female Age: 15–17 years Race: unspecified NZ Location: New Zealand |
91 | Spine BMD Trochanter BMD Femoral neck BMD |
1.5 % greater increase in trt (P < 0.05) 4.6 % greater increase in trt 4.8 % greater increase in trt |
||
| 330 mL UHT milk (560 mg calcium), 330 mL UHT milk + 200 or 320 IU vitamin D3, or control | Du et al. 2004 [170] | 2-year school-based randomized trial | Sex: female Age: 10–12 years Race: Chinese Location: Beijing, China |
757 | Total body BMC Bone area |
35.9 % (P = 0.03) 31.3 % greater increase in trt (P = 0.2) |
||
95 % CI 95 % confidence interval, BMC bone mineral content, BMD bone mineral density, CSA cross-sectional area, NS not significant, pQCT peripheral quantitative computed tomography, RCT randomized controlled trial, SSI stress–strain index, trt treatment, UHT ultra-heat-treated, vBMD volumetric bone mineral density
aSignificantly greater than the control
Table 5.
Calcium and exercise and bone health in children and adolescents
| Reference | Study description | Population description | Number of subjects | End points | Results | |
|---|---|---|---|---|---|---|
| Molgaard et al. 2001 [245] | 1-year prospective observational study to determine effects of dietary calcium and physical activity on bone changes. | Sex: 140 boys, 192 girls Age: 5–19 years at baseline Race: Caucasian Location: Copenhagen, Denmark |
332 | Correlation with calcium intake, r | Correlation with physical activity, r | |
| Whole bone area gain adjusted for height and weight | 0.03, girls | 0.28 | ||||
| −0.34, boys | 0.28 | |||||
| Total body BMC gain adjusted for bone area, height, and weight | 0.21, girls | −0.06 | ||||
| 0.34, boys | −0.04 | |||||
| Carter et al. 2001 [171] | Cross-sectional study to investigate the relationship between calcium intake and BMC. | Sex: 108 boys and 119 girls Mean age: 13 years Race: Primarily Caucasian Location: Saskatoon Canada |
227 | Total body BMC | No association | |
| Lumbar spine BMC | No association | |||||
| Lloyd et al. 2000 [174] | 6-year prospective study to determine effects of dietary calcium and physical activity on bone changes | Sex: girls Age: 12–18 years at baseline Race: Caucasian Location: Pennsylvania |
81 | Total body BMC gain | NS | |
| Total body BMD gain | NS | |||||
| Femoral neck BMDa | r = 0.42 with exercise | |||||
| Lappe et al. 2014 [172] | 6-year prospective study of calcium intake and physical activity on bone accrual | Sex: boys and girls Age: 5–16 years at baseline Race: white, black, Asian, Hispanic, and non-Hispanic Location: 5 sites in the USA |
1743 | Mixed-model analyses | ||
| Total body BMC gaina | Associated with physical activity in nonblacks | |||||
| Spine BMC gaina | Associated with physical activity in blacks and nonblack males | |||||
| Total hip BMC gaina | Associated with calcium in nonblack females | |||||
| Adjusted for changes in height, age, and baseline BMC | Associated with physical activity in nonblacks and black males | |||||
BMC bone mineral content, BMD bone mineral density, NS not significant
aSignificantly greater than the control
Table 6.
Vitamin D and bone health in children and adolescents
| Reference | Study description | Population description | Number of subjects | Primary end points | Results | ||||
|---|---|---|---|---|---|---|---|---|---|
| RCTs | |||||||||
| Du et al. 2004 [170] | This study was a 2-year milk intervention trial in girls from nine primary schools in Beijing. Schools were randomized into three groups: (1) a carton of 330-mL milk fortified with calcium per school day, (2) a carton of 330-mL milk fortified with 200 or 320 IU 8 mg vitamin D3, and (3) control | Sex: female Age: 10–12 years Race: Asian (Chinese) Location: Beijing, China Year(s): 1999–2001 Baseline 25(OH)D as mean (SD) in nmol/L: Control: 19.1 (7.4) Calcium only: 17.7 (8.7) Calcium + vitamin D: 20.6 (8.8) |
757 | Data are shown for a subsample with bone measures (N = 346) | Percent change | P | |||
| Milk with calcium (n = 111) | Milk with calcium + vitamin D (n = 113) | Control (n = 122) | |||||||
| Total body | |||||||||
| Bone area | 29.5 | 28.5 | 31.3 | NS | |||||
| BMC | 38.4 | 39.7 | 35.9 | <0.01 | |||||
| Percent change, calcium + vitamin D, minus calcium | P | Percent change, calcium + vitamin D, minus control | P | ||||||
| Total body | |||||||||
| Bone area | 0.8 | NS | −1.8 | 0.04 | |||||
| BMC | −0.8 | NS | 2.6 | <0.01 | |||||
| Size-adjusted BMC | 1.3 | <0.01 | 2.4 | <0.01 | |||||
| • Mean percent change values in the calcium + vitamin D group were significantly different from controls. • Adjusted percentage difference in change values are adjusted for baseline. • Size-adjusted BMC is adjusted for baseline BMC, bone area, height, weight, and menstrual status. | |||||||||
| Cheng et al. 2005 [159] | The purpose of this study was to examine the effects of both food-based and pill supplements of calcium and vitamin D3 on bone mass and body composition. Children were randomized into four groups: (1) 1000 mg calcium + 200 IU vitamin D3, (2) 1000 mg calcium + vitamin D3 placebo, (3) 1000 mg calcium from dairy products,and (4) calcium + vitamin D3 placebo for 2 years | Sex: female Age: 10–12 years Race: Finnish, otherwise unspecified Location: Jyvaskyla and surrounding cities in Central Finland Year(s): Unspecified Baseline 25(OH)D as mean (95 % CI): 45.9 (43.8, 48.0) nmol/L |
195 | Data are shown for the overall group who started the intervention (N = 181) | Percent change | P | |||
| Calcium (n = 41) | Calcium + vitamin D3 (n = 46) | Cheese (n = 39) | Placebo (n = 39) | ||||||
| Lumbar spine | |||||||||
| Bone area | 28.4 | 23.0 | 25.3 | 23.4 | NS | ||||
| BMC | 24.0 | 46.9 | 52.4 | 47.0 | NS | ||||
| Total hip | |||||||||
| Bone area | 24.1 | 17.0 | 18.1 | 17.3 | NS | ||||
| BMC | 20.3 | 33.6 | 36.9 | 33.6 | NS | ||||
| Femoral neck | |||||||||
| Bone area | 3.8 | 12.6 | 13.1 | 15.0 | NS | ||||
| BMC | 3.3 | 24.0 | 26.5 | 22.4 | NS | ||||
| Radius | |||||||||
| CSA | 26.0 | 23.0 | 26.2 | 21.3 | NS | ||||
| BMC | 24.4 | 22.6 | 25.9 | 22.2 | NS | ||||
| vBMD | 2.6 | 3.4 | 3.1 | 2.0 | NS | ||||
| Polar moment of inertia | 62.0 | 53.3 | 61.8 | 51.8 | NS | ||||
| Tibia | |||||||||
| CSA | 15.8 | 15.6 | 15.9 | 14.8 | NS | ||||
| BMC | 24.3 | 23.0 | 25.2 | 22.7 | NS | ||||
| vBMD | 7.5 | 6.9 | 8.3 | 7.8 | NS | ||||
| Cortical bone thickness | 29.8 | 31.7 | 37.1 | 31.1 | NS | ||||
| Polar moment of inertia | 41.5 | 41.3 | 42.6 | 39.7 | NS | ||||
| • Data presented above show no interaction between the groups with the intent-to-treat analysis. • There was a significant interaction with cortical thickness of the tibia between the groups (P < 0.05) where this measure increased more in the cheese group with a compliance >50 % versus placebo (P = 0.01), calcium + vitamin D (P < 0.01), or calcium (P = 0.04). • Bonferroni correction for multiple comparisons was applied to these analyses. | |||||||||
| El-Hajj Fuleihan et al. 2006 [175] | Musculoskeletal responses were determined in response to weekly doses of vitamin D3 (1400 and 14,000 IU) over 1 year | Sex: female Age: 10–17 years Race: Lebanese, otherwise unspecified Location: greater Beirut, Lebanon area Year(s): 2001–2003 Baseline 25(OH)D as mean (SD): 14 (8) ng/ml |
179 | Data are shown for the overall group (N = 168) | Percent change | P | |||
| High-dose treatment, 14,000 IU/week (n = 55) | Low-dose treatment, 1400 IU/week (n = 58) | Placebo (n = 55) | |||||||
| Lumbar spine | |||||||||
| Bone area | 4.3 | 5.0 | 4.0 | NS | |||||
| BMC | 12.9 | 14.5 | 10.8 | NS | |||||
| Total hip | |||||||||
| Bone area | 5.7 | 4.0 | 2.4 | <0.01 | |||||
| BMC | 12.8 | 11.2 | 7.8 | 0.02 | |||||
| Femoral neck | |||||||||
| Bone area | 0.8 | 0.0 | 0.7 | NS | |||||
| BMC | 5.2 | 4.4 | 3.9 | NS | |||||
| Trochanter | |||||||||
| Bone area | 7.8 | 6.8 | 4.7 | NS | |||||
| BMC | 14.2 | 13.6 | 9.4 | NS | |||||
| Total body | |||||||||
| Bone area | 6.2 | 6.1 | 5.0 | NS | |||||
| BMC | 12.0 | 11.3 | 8.7 | NS | |||||
| Lean mass | 9.0 | 8.7 | 5.7 | 0.05 | |||||
| • In premenarchal girls, trochanter BMC and lean mass reached significance. • Postmenarchal girls did not exhibit significant changes in any parameters of interest. | |||||||||
| Viljakainen et al. 2006 [177] | This study sought to determine the effect of vitamin D3 supplementation (200 or 400 IU/day) for 1 year on bone mineral augmentation in adolescent girls with adequate dietary calcium intake. | Sex: female Age: 11.4 ± 0.4 years Race: white Location: Helsinki, Finland Year(s): 2001–2003 Baseline 25(OH)D as mean (SD) in nM: Placebo: 47.8 (18.2) 5 μg/day: 46.3 (17.4) 10 μg/day: 46.7 (16.2) |
228 | Data are shown for the overall group (N = 212) | Mean change | P | |||
| High-dose treatment, 400 IU/day (n = 74) | Low-dose treatment, 200 IU/day (n = 65) | Placebo (n = 73) | |||||||
| Lumbar spine | |||||||||
| Bone area (cm2) | 3.8 | 4.1 | 3.5 | NS | |||||
| BMC (g) | 6.2 | 5.9 | 5.1 | NS | |||||
| Femur | |||||||||
| Bone area (cm2) | 2.0 | 2.2 | 2.4 | NS | |||||
| BMC (g) | 3.3 | 3.4 | 3.2 | NS | |||||
| • Data presented above are for the intent-to-treat analysis. • In the compliance-based analysis only in the entire sample, vitamin D supplementation vs placebo increased femoral BMC augmentation by 14.3 % with 200-IU doses and by 17.2 % with 400-IU doses (P = 0.012) • In the compliance-based analysis only in the entire sample, a dose–response effect was observed at the lumbar spine with the 400-IU (P = 0.039), but not the 200-IU dose. • In a subanalysis based on puberty (n = 111) and using a compliance-based analysis only, adjusted for Tanner stage, increase in bone area, and weight gain, there was a dose-dependent and significant difference in the lumbar spine BMC augmentation at midpuberty (P = 0.01), but not at early puberty. | |||||||||
| Andersen et al. 2008 [180] | The aim of this study was to assess the effect of supplemental vitamin D on bone status in Pakistani immigrants. This 1-year intervention with vitamin D3 (10 and 20 μg /day) included girls (10.1–14.7 years), women (18.1–52.7 years), and men (17.9–63.5 years) of Pakistani origin living in Denmark | Sex: male and female Age: 10–63 years Race: of Pakistani origin Location: Copenhagen area, Denmark Year(s): 2002–2003 Baseline 25(OH)D as median (25th, 75th percentiles) in nmol/L: Placebo: 7.3 (5.3, 23.6) 10 μg/day: 16.9 (12.1, 21.1) 20 μg/day: 8.8 (5.2, 17.1) |
247 | Data are shown for the girls group only (n = 26) | Median value | P | |||
| 20 μg/day (n = 9) | 10 μg/day (n = 9) | Placebo (n = 8) | |||||||
| Baseline | |||||||||
| Total body | |||||||||
| Bone area (cm2) | 1633 | 1497 | 1760 | NS | |||||
| BMC (g) | 1473 | 1308 | 1666 | NS | |||||
| Lumbar spine | |||||||||
| Bone area (cm2) | 39.9 | 41.2 | 43.2 | NS | |||||
| BMC (g) | 30.0 | 28.3 | 33.9 | NS | |||||
| 1 year | |||||||||
| Total body | |||||||||
| Bone area (cm2) | 1784 | 1669 | 1906 | NS | |||||
| BMC (g) | 1625 | 1595 | 1923 | NS | |||||
| Lumbar spine | |||||||||
| Bone area (cm2) | 42.7 | 42.7 | 46.5 | NS | |||||
| BMC (g) | 39.5 | 35.6 | 41.9 | NS | |||||
| Data presented above indicate no significant differences between groups at baseline (nonparametric ANOVA) and no effect of the intervention on bone indices. | |||||||||
| Khadilkar et al. 2010 [178] | This study investigated the effect of quarterly doses of vitamin D2 supplementation (300,000 IU or 7.5 mg) on BMC in underprivileged adolescent girls over 1 year | Sex: female Age: 14–15 years Race: Indian Location: Pune, India Year(s): 2006–2007 Baseline 25(OH)D as median (25th percentile–75th percentile): Vitamin D + calcium group: 24.5 (12.7–33.2) nmol/L Placebo + calcium group: 20.8 (12.7–30.4) nmol/L |
50 | Data are shown for the overall group (N = 50) | Percent change | P | |||
| Vitamin D + calcium (n = 25) | Placebo + calcium (n = 25) | ||||||||
| Total body | |||||||||
| Bone area | 5.1 | 3.6 | NS | ||||||
| BMC | 10.1 | 8.2 | NS | ||||||
| Lumbar spine | |||||||||
| Bone area | 3.3 | 3.9 | NS | ||||||
| BMC | 10.5 | 11.3 | NS | ||||||
| • Data presented above are unadjusted median percent change values. • After adjusting for age (current), height, weight, lean body mass, initial dietary calcium, and calcium compliance, there was a significant increase in the size and compliance adjusted total BMC and bone area change in subjects who were within 2 years of menarche versus those less than 2 years since menarche (both P ≤ 0.04). | |||||||||
| Molgaard et al. 2010 [173] | This study investigated the effect of vitamin D3 supplementation (5 or 10 μg/day) for 1 year on bone mass and bone turnover, as well as the possible influence of VDR and ER genotype on the effect of the supplementation | Sex: female Age: 10–11 years Race: Danish-born citizens, otherwise unspecified Location: Copenhagen and Frederiksberg, Denmark Year(s): 2001–2003 Baseline 25(OH)D as mean (SD) in nmol/L: Placebo: 43.4 (17.1) 5 μg/day: 41.9 (17.6) 10 μg/day: 44.4 (16.6) |
225 | Data are shown for the overall group (N = 225) | Mean change | P | |||
| High-dose treatment, 400 IU/day (n = 75) | Low-dose treatment, 200 IU/day (n = 75) | Placebo (n = 75) | |||||||
| Total body | |||||||||
| Bone area (cm2) | 195 | 189 | 188 | NS | |||||
| BMC (g) | 249 | 245 | 241 | NS | |||||
| Lumbar spine | |||||||||
| Bone area (cm2) | 4.9 | 5.5 | 5.6 | 0.04 | |||||
| BMC (g) | 7.4 | 8.1 | 8.2 | NS | |||||
| • Lumbar spine bone area in the high-dose group differed significantly from placebo. • Vitamin D supplementation increased total body BMC (P = 0.048) in the FF VDR genotype but not in the Ff or ff VDR genotypes. | |||||||||
| Ward et al. 2010 [179] | This study aimed to determine the effect of vitamin D2 supplementation (4 doses of 150,000 IU given over 1 year) on the adolescent musculoskeletal system determined by DXA and peripheral quantitative computed tomography. | Sex: female Age: 12–14 years Race: multiethnic, primarily South Asian Location: Manchester, UK Year(s): 2006–2007 Baseline 25(OH)D as mean (SD) in nmol/L: Placebo: 17.9 (7.4) Vitamin D: 18.1 (8.0) |
73 | Data are shown for the overall group (N = 72) | Mean change | P | |||
| Vitamin D | Placebo | ||||||||
| Lumbar spine | |||||||||
| Bone area (cm2) | 0.20 | 0.15 | NS | ||||||
| BMC (g) | 0.52 | 0.57 | NS | ||||||
| Radius 4 % | |||||||||
| Total BMD (mg/cm3) | 21.3 | 13.01 | NS | ||||||
| Trab BMD (mg/cm3) | 3.07 | 3.23 | NS | ||||||
| CSA_4 (mm2) | 6.19 | 9.27 | NS | ||||||
| Radius 50 % | |||||||||
| CSMA (mm2) | −29.4 | 47.1 | NS | ||||||
| CSA_50 (mm2) | −0.67 | −1.91 | NS | ||||||
| Ct BMC (mg/mm) | 2.73 | 3.12 | NS | ||||||
| Ct BMD (mg/cm3) | 17.4 | 23.9 | NS | ||||||
| Ct area (mm2) | 1.26 | 1.28 | NS | ||||||
| Cort thk (mm) | 0.10 | 0.12 | NS | ||||||
| SSI (mm3) | 3.02 | 6.91 | NS | ||||||
| Tibia 4 % | |||||||||
| Total BMD (mg/cm3) | 10.5 | 9.79 | NS | ||||||
| Trab BMD (mg/cm3) | 9.31 | 7.00 | NS | ||||||
| CSA_4 (mm2) | −1.19 | −3.28 | NS | ||||||
| Tibia 66 % | |||||||||
| CSMA (mm2) | 161.2 | 197.6 | NS | ||||||
| CSA_66 (mm2) | 17.09 | 7.70 | NS | ||||||
| Ct BMC (mg/mm) | 7.68 | 9.66 | NS | ||||||
| Ct BMD (mg/cm3) | 14.26 | 13.98 | NS | ||||||
| Ct area (mm2) | 3.98 | 5.85 | |||||||
| Cort thk (mm) | −0.02 | 0.09 | |||||||
| SSI (mm3) | 116.3 | 85.15 | |||||||
|
• P values presented above are adjusted for baseline and follow-up weight, follow-up height, and baseline bone measurement. • There were no effects of vitamin D supplementation on bone. | |||||||||
| Al-Shaar et al. 2013 [176] | This study investigated the impact of weekly vitamin D3 (1400 and 14,000 IU) on hip geometric dimensions over 1 year. The images were acquired from DXA-derived hip structural analyses | Sex: male and female Age: 10–17 years Race: Lebanese, otherwise unspecified Location: greater Beirut, Lebanon area Year(s): 2001–2003 Baseline 25(OH)D as medians [interquartile range]: Overall, girls: 11.9 [9.1–16.6] ng/ml Overall, boys: 15.5 [6.0–44.9] ng/ml |
338 | Data are shown for females only (n = 167) | Percent change | P | |||
| High-dose treatment, 14,000 IU/week (n = 54) | Low-dose treatment, 1400 IU/week (n = 58) | Placebo (n = 55) | |||||||
| Narrow neck | |||||||||
| CSA | 8.1 | 8.3 | 8.2 | NS | |||||
| Outer diameter | 2.0 | 0.8 | 2.8 | 0.02 | |||||
| Section modulus | 11.4 | 11.3 | 11.7 | NS | |||||
| Buckling ratio | −4.2 | −6.5 | −2.0 | 0.05 | |||||
| Shaft | |||||||||
| CSA | 10.4 | 11.6 | 9.9 | NS | |||||
| Outer diameter | 2.1 | 2.3 | 1.4 | NS | |||||
| Section modulus | 12.5 | 13.5 | 11.0 | NS | |||||
| Buckling ratio | −7.2 | −8.4 | −8.9 | NS | |||||
| Intertrochanter | |||||||||
| CSA | 8.5 | 9.5 | 7.1 | NS | |||||
| Outer diameter | 2.5 | 1.3 | 1.9 | NS | |||||
| Section modulus | 12.7 | 13.1 | 10.1 | NS | |||||
| Buckling ratio | −3.0 | −5.6 | −3.6 | NS | |||||
| • Data presented above were adjusted for baseline height, change in lean mass and height, sun exposure, physical activity, calcium intake, and menarchal status. • Boys did not exhibit significant changes in any parameters of interest. • A dose effect was not detected and no beneficial effect of vitamin D was observed by pubertal stage. | |||||||||
| Prospective studies | |||||||||
| Breen et al. 2011 [181] | This study sought to determine relationships among 25(OH)D, IGF-I, and bone in prepubertal females over a period of up to 9 years (median of 5 years) | Sex: female Age: 4–8 years Race: white and black Location: Georgia, USA Year(s): 1997–2008 |
76 | Data are shown for the overall group (N = 76) | Combined 25(OH)D + IGF-I | 25(OH)D | IGF-I | ||
| BMC | |||||||||
| Total body | 0.84 | 0.81 | 0.87 | ||||||
| Lumbar spine | 0.72 | 0.70 | 0.76 | ||||||
| Proximal femur | 0.81 | 0.77 | 0.85 | ||||||
| Forearm | 0.78 | 0.76 | 0.81 | ||||||
| • Data are presented above as R
2 and the linear mixed models regressed BMC on age, IGF-I, 25(OH)D, season, and race, and interactions among these variables and show that IGF-I was more strongly associated with BMC accrual than 25(OH)D at all sites • The rate of BMC accrual was negatively associated with 25(OH)D. When IGF-I and 25(OH)D were included in the same regression equation, 25(OH)D did not have a significant predictive effect on BMC accrual above and beyond that of IGF-I. | |||||||||
| Cross-sectional studies | |||||||||
| Cheng et al. 2003 [182] | Associations of serum 25(OH)D with BMC at different bone sites were measured by DXA and pQCT. | Sex: female Age: 10–12 years Race: Finnish, otherwise unspecified Location: Jyvaskyla, Finland Year(s): 1999–2000 |
193 | Data are shown for the overall group (N = 193) | Deficient, ≤25 nmol/L (n = 61) | Insufficient, 26–40 nmol/L (n = 89) | Sufficient, >40 nmol/L (n = 43) | P | |
| Total body | |||||||||
| Bone area (cm2) | 1468 | 1475 | 1436 | NS | |||||
| BMC (g) | 1380 | 1414 | 1347 | NS | |||||
| Femur | |||||||||
| Bone area (cm2) | 23.9 | 23.7 | 23.1 | NS | |||||
| BMC (g) | 19.7 | 20.4 | 18.9 | 0.05 | |||||
| Femoral neck | |||||||||
| Bone area (cm2) | 3.837 | 3.84 | 3.67 | NS | |||||
| BMC (g) | 3.21 | 3.30 | 3.13 | NS | |||||
| Lumbar spine | |||||||||
| Bone area (cm2) | 27.5 | 28.1 | 27.3 | NS | |||||
| BMC (g) | 22.6 | 23.9 | 21.9 | NS | |||||
| Distal radius | |||||||||
| Whole bone CSA (mm2) | 241 | 226 | 209 | <0.01 | |||||
| Whole bone vBMD (mg/cm3) | 273 | 292 | 298 | <0.01 | |||||
| Trabecular vBMD (mg/cm3) | 221 | 229 | 231 | NS | |||||
| Tibia shaft | |||||||||
| Whole bone CSA (mm2) | 368 | 372 | 363 | NS | |||||
| Whole bone vBMD (mg/cm3) | 848 | 865 | 853 | 0.02 | |||||
| Trabecular vBMD (mg/cm3) | 525 | 534 | 524 | NS | |||||
| • Data are adjusted for Tanner stage and BMI. • P value is shown with Bonferroni adjustment in the ANOVA for multiple comparisons. • Sufficient is different from the insufficient group in BMC of the femur (P = 0.04). • Sufficient is different from the deficient group in whole bone CSA and vBMD of the radius (P < 0.01). • Insufficient is different from the deficient group in whole bone CSA (P = 0.05) and vBMD (P < 0.01) of the radius and whole bone vBMD (P < 0.02) of the tibia. | |||||||||
| Foo et al. 2009 [183] | This cross-sectional study investigated the influence of low vitamin D status on bone mass, bone turnover, and muscle strength. | Sex: female Age: 15 years Race: Chinese, otherwise unspecified Location: Beijing, China Year(s): 2004 |
301 | Data are shown for the overall group (N = 301) | Severe deficiency, <25 nmol/L (n = 94) | Deficiency, ≤50 nmol/L (n = 174) | Sufficient, >50 nmol/L (n = 33) | P trend | |
| Size-adjusted BMC (g) | |||||||||
| Total body | 2230 | 2291 | 2417 | <0.01 | |||||
| Proximal forearm | 1.44 | 1.47 | 1.53 | <0.01 | |||||
| Distal forearm | 1.10 | 1.52 | 1.86 | <0.01 | |||||
| Data are presented as means adjusted for pubertal breast stage, handgrip muscle strength, organized sports participation, physical activity level, dietary vitamin D, and calcium. | |||||||||
| Lee et al. 2013 [184] | Risk factors were evaluated for low 25(OH)D status and its relationships with bone health | Sex: male and female Age: 9.3 ± 1.9 years Race: Korean, otherwise unspecified Location: Seoul and Gyeonggi-do, South Korea Year(s): unspecified |
100 | Data are shown for the overall group (N = 100) | Total body BMC | P | |||
| Unadjusted | 0.028 | 0.08 | |||||||
| Adjusted model 1 | 0.02 | 0.07 | |||||||
| Adjusted model 2 | 0.02 | 0.04 | |||||||
| • Data presented above are β scores correlating 25(OH)D with BMC and corresponding P values using multiple linear regression analyses • Model 1 is adjusted for sex, puberty, fat mass, and lean mass • Model 2 is adjusted for model 1 variables + physical activity and calcium intake | |||||||||
25(OH)D 25-hydroxyvitamin D, 95 % CI 95 % confidence interval, ANOVA analysis of variance, BMC bone mineral content, Cort thk cortical thickness, CSA cross-sectional area, CSMA cross-sectional moment of inertia, Ct cortical, DXA dual-energy x-ray absorptiometry, IGF insulin-like growth factor, NS not significant, RCT randomized controlled trial, SSI stress–strain index, Trab trabecular, vBMD volumetric bone mineral density, VDR vitamin D receptor
Table 7.
Other micronutrients and bone health in children and adolescents
| Micronutrient | Reference | Study description | Population description | Number of subjects | End points | Results | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RCTs | |||||||||||
| Magnesium | Carpenter et al. 2006 [185] | 1-year placebo-controlled, double-blind RCT of 300 mg/day supplementation of MgO | Sex: female Age: 8–14 years Race: white Location: New Haven, CT |
44 | BMC of total hip, femoral neck, Ward’s area, and lumbar spine | Combined overall hip | |||||
| Treatment | Placebo | ||||||||||
| 1.05 % | 0.97 %, P = 0.0534 | ||||||||||
| Prospective studies | |||||||||||
| Fluoride | Levy et al. 2009 [187] | Lifetime associations of average daily fluoride intake and DXA bone outcomes at age 11 years | Sex: male and female Age: birth to 11 years for fluoride assessments; mean age 11.2 years for DXA assessment Race: 97 % white Location: Iowa Year(s): recruitment took place from 1992 to 1995 (fluoride study) and from 1998 to 2000 (bone development study) |
481 | BMC | Hip | Spine | Total body less head | |||
| Girls | |||||||||||
| 0–11 years | 0.07, NS | 0.17, NS | 0.10, NS | ||||||||
| 0–8.5 years | 0.04, NS | 0.13, NS | 0.08, NS | ||||||||
| 0–3 years | 0.03, NS | 0.06, NS | 0.05, NS | ||||||||
| 3–6 years | −0.01, NS | 0.05, NS | 0.02, NS | ||||||||
| 6–8.5 years | 0.11, NS | 0.19, 0.01 | 0.16, NS | ||||||||
| 85.5–11 years | 0.18, 0.01 | 0.24, <0.01 | 0.19, NS | ||||||||
| Boys | |||||||||||
| 0–11 years | 0.21, 0.01 | 0.23, 0.01 | 0.23, 0.01 | ||||||||
| 0–8.5 years | 0.21, 0.01 | 0.23, 0.01 | 0.24, 0.01 | ||||||||
| 0–3 years | 0.22, 0.01 | 0.22, 0.01 | 0.23, 0.01 | ||||||||
| 3–6 years | 0.14, NS | 0.18, NS | 0.19, 0.01 | ||||||||
| 6–8.5 years | 0.09, NS | 0.12, NS | 0.10, NS | ||||||||
| 85.5–11 years | 0.14, NS | 0.14, NS | 0.15, NS | ||||||||
| • Data are from unadjusted bivariate associations (r) with fluoride intake and bone outcomes and corresponding P value. • No statistically significant relationships between daily fluoride intake and bone measures were found in adjusted models (for age, height, weight, and Tanner stage). | |||||||||||
| Fluoride | Levy et al. 2014 [188] | Lifetime associations of average daily fluoride intake and DXA bone outcomes at age 15 years | Sex: male and female Age: birth to 15 years for fluoride assessments; mean age 15.3 years for DXA assessments Race: 98 % white Location: Iowa Year(s): recruitment took place from 1992 to 1995 (fluoride study) and from 1998 to 2000 (bone development study) |
358 | BMC | Females | Males | ||||
| ß | R 2 | Partial R 2 | ß | R 2 | Partial R 2 | ||||||
| Total body less head | 234, 0.02 | 0.03 | 0.03 | 182, NS | 0.02 | 0.02 | |||||
| Spine | 7.32, 0.04 | 0.03 | 0.03 | 5.24, NS | 0.02 | 0.02 | |||||
| Hip | 2.92, NS | 0.01 | 0.01 | 1.79, NS | <0.01 | <0.01 | |||||
| • Data are from unadjusted bivariate associations (β) with fluoride intake and bone outcomes and corresponding P value. • With adjustment for height, weight, time since PHV, Tanner stage, calcium intake, and physical activity, none of the associations remained statistically significant. • No significant differences were observed between fluoride intake and bone measures across tertiles of fluoride intake from birth to 15 years. | |||||||||||
| Observational studies | |||||||||||
| Vitamin K | O’Connor et al. 2007 [191] | Cross-sectional associations of undercarboxylated osteocalcin (%ucOC) as an index of vitamin K status and BMC | Sex: female Age: 11–12 years Race: presumed white Location: Denmark |
223 | Serum %ucOC and total body BMC | β = −0.045, P = 0.016 | |||||
| Lumbar BMC | β = −0.055, P = 0.037 | ||||||||||
| Sodium | Hoppe et al. 2000 [149] | Cross-sectional analysis of nutrient intakes determined by 7-day diet records and bone | Sex: half were female, half were male Age: 10 years Race: presumed white Location: Denmark Year(s): 1997–1998 |
105 | Total body BMC | r = −0.206, P < 0.05 | |||||
| Anterior–posterior projected bone area (cm2) | r = −0.215, P < 0.05 | ||||||||||
| Phosphorous | Hoppe et al. 2000 [149] | Cross-sectional analysis of nutrient intakes determined by 7-day diet records and bone | Sex: half were female and half were male Age: 10 years Race: presumed white Location: Denmark Year(s): 1997–1998 |
105 | Total body BMC | r = −0.297, P < 0.01 | |||||
| Anterior–posterior projected bone area (cm2) | r = −0.284, P < 0.01 | ||||||||||
| Vitamin C | Prynne et al. 2006 [189] | Cross-sectional Cambridge Bone Studies to relate fruit and vegetable and nutrient intake from 7-day food diaries in 5 age and sex cohorts | Sex: female and male, nearly half of each Age: 16–19 years Race: presumed white Location: UK |
257 | Percent change with doubling in vitamin C intake from univariate analysis, in 4th-grade girls only | ||||||
| Boys | Girls | ||||||||||
| Total body BMC | 5.5, P < 0.01 | 1.4, NS | |||||||||
| Spine BMC | 5.6, P < 0.05 | 2.1, NS | |||||||||
| Total hip BMC | 5.7, P < 0.05 | −0.05, NS | |||||||||
| Femoral neck BMC | 5.4, P < 0.01 | 5.2, NS | |||||||||
| Trochanter BMC | 5.8, P < 0.05 | 2.5, NS | |||||||||
| Vitamin C, zinc, and iron | Laudermilk et al. 2012 [190] | Cross-sectional analysis of nutrient intakes determined by the Harvard Youth/Adolescent Food frequency Questionnaire | Sex: female Age: 8–12 years, 4th- and 6th-grade students Race: ~87 % white, 7 % Asian, 3 % black, 2 % Latino and 1 % Native Hawaiian Location: Tucson, Arizona |
453 (n = 184 4th graders, n = 179 6th graders) |
pQCT | Vitamin C | Zinc | ||||
| Femur 20 % site | |||||||||||
| Cortical density | NS | 0.16, P < 0.05 | |||||||||
| Periosteal circumference | 0.17, P < 0.05 | NS | |||||||||
| Endosteal circumference | 0.17, P < 0.05 | NS | |||||||||
| SSI | 0.18, P < 0.05 | NS | |||||||||
| Tibia 4 % site | |||||||||||
| Trabecular area | 0.18, P < 0.05 | NS | |||||||||
| Periosteal circumference | 0.19, P < 0.01 | NS | |||||||||
| Tibia 66 % site | |||||||||||
| Cortical density | NS | 0.15, P < 0.05 | |||||||||
| Cortical area | 0.15, P < 0.05 | NS | |||||||||
| SSI | 0.18, P < 0.05 | NS | |||||||||
| In regression modeling, iron was negatively associated with femoral cortical area and tibia SSI. | |||||||||||
| Fluoride | Grobler 2009 [186] | This field study included the whole population of children aged 10–15 years living in areas of high and low fluoride in the drinking water. | Sex: male and female Age: 10–15 years Race: not specified, but of mixed ethnicity (i.e., from Khoi, Caucasian, and Negroid roots that developed into a homogenous ethnic group over many years) Location: South Africa Year(s): not specified |
166 (n = 77 from a 0.19 mg/L F area; n = 89 from a 3.00 mg/L F area) |
High fluoride | Low fluoride | P | ||||
| Girls | Boys | Girls | Boys | ||||||||
| Radius BMC (g) | |||||||||||
| 10–11 years | 1.29 | 1.29 | 1.26 | 1.32 | NS | ||||||
| 12–13 years | 1.56 | 1.41 | 1.33 | 1.29 | <0.05a | ||||||
| 14–15 years | 1.80 | 1.80 | 1.18 | 1.52 | <0.05a | ||||||
| Radius bone width (cm) | |||||||||||
| 10–11 years | 0.97 | 0.98 | 0.98 | 1.01 | NS | ||||||
| 12–13 years | 1.09 | 1.08 | 1.04 | 1.15 | NS | ||||||
| 14–15 years | 1.10 | 1.14 | 1.18 | 1.21 | NS | ||||||
%ucOC percentage of undercarboxylated osteocalcin, BMC bone mineral content, DXA dual-energy x-ray absorptiometry, NS not significant, PHV peak height velocity, pQCT peripheral quantitative computed tomography, RCT randomized controlled trial, SSI stress–strain index
aSignificant differences in BMC in the 12- to 13-year-olds and the 14- to 15-year-olds were found among boys from the high fluoride area and girls from the low fluoride area
Table 8.
Food patterns and bone health in children and adolescents
| Food | Source | Study description | Population description | Number of subjects | End points | Results | |||
|---|---|---|---|---|---|---|---|---|---|
| RCTs | |||||||||
| Dairy | Du et al. 2004 [170] | 2-year school-based randomized trial of 330 mL milk, 330 mL milk + 5 or 8 μg vitamin D3, or control | Sex: female Age: 10–12 years Race: Chinese Location: Beijing, China |
757 | Group mean increase | P | |||
| Treatment | Placebo/control | ||||||||
| Height | 0.95 % | 0.087 % | <0.0005 | ||||||
| Total body BMC | 38.4 % | 35.9 % | 0.03 | ||||||
| Bone area | 29.5 % | 31.3 % | 0.2 | ||||||
| Dairy | Courteix et al. 2005 [158] | 12-month randomized, double-blind, placebo-controlled study Baseline calcium: supplement = 1008 (398); placebo = 988 (345) Was combined with an exercise intervention that found a combined effect not reported here. After randomization, fewer subjects were in the dairy group than in the placebo group (34 vs 79, respectively, at the baseline). It was expected that 240 subjects would be recruited, but because of the publicity surrounding Mad Cow disease, many parents were afraid of dairy products. |
Sex: premenarchal female Age: 8–13 years Race: Caucasian Location: France |
113 | Calcium supplement | Placebo | |||
| Total body | BMC | 155 ± 79 | 166 ± 66 | ||||||
| Lumbar spine | BMC | 3.429 ± 2.388 | 3.228 ± 2.642 | ||||||
| Femoral neck | BMC | 0.248 ± 0.187 | 0.185 ± 0.103 | ||||||
| Trochanter | BMC | 0.941 ± 0.525 | 0.796 ± 0.582 | ||||||
| Wards | BMC | 0.001 ± 0.054 | 0.047 ± 0.086 | ||||||
| Ultradistal radius | BMC | 0.104 ± 0.091 | 0.111 ± 0.107 | ||||||
| Mid radius | BMC | 0.305 ± 0.230 | 0.355 ± 0.322 | ||||||
| 1/3 distal | BMC | 0.072 ± 0.078 | 0.093 ± 0.077 | ||||||
| Adjusted for lean tissue mass. All NS | |||||||||
| Dairy | Cheng et al. 2005 [159] | 2 year double-blind, placebo-controlled RCT of calcium (1000 mg) + vitamin D3 (200 IU), calcium (1000 mg), cheese (1000 mg calcium), and placebo | Sex: female Age: 10–12 years Race: presumed white Location: Finland |
195 | Cheese | P | |||
| Total body | BMD (%) | 10.4 | 8.9 (compliance >50) | 0.044 | |||||
| Femoral neck | BMC (%) | 26.5 | 22.4 | NS | |||||
| Total femur | BMC (%) | 36.9 | 33.6 | NS | |||||
| Spine | BMC (%) | 52.4 | 47.0 | NS | |||||
| Tibia cortical thickness | pQCT (%) | 37.1 | 31.1 (compliance 50) | 0.01 | |||||
| Dairy | Merrilees et al. 2000 [169] | 2-year RCT of dairy food supplementation and 1-year follow-up after cessation of intervention | Sex: female Age: 15–17 years Race: presumed white Location: New Zealand Stratified by forearm BMD at baseline |
91 | Total body | BMC (g) | 168.9 | 167.4 | NS |
| Lumbar spine | BMC (g) | 3.83 | 2.58 | NS | |||||
| Femoral neck | BMC (g) | 0.12 | 0.06 | NS | |||||
| Trochanter | BMC (g) | 0.75 | 0.25 | <0.05 | |||||
| Difference disappeared 1 year after cessation | |||||||||
| Fiber | Abrams et al. 2005 [193] | 1-year placebo-controlled RCT of 8 g/day mixed short and long inulin-type fructans | Sex: half were male and half were female Age: 9–13 years Tanner stage 2 or 3 Race: 53 % white, 14 % black, 22 % Hispanic, 10 % Asian Location: Houston, TX Between 5th and 95th percentile BMI |
100 | Total body | BMC, % | 18.3 | 16.7 | 0.03 |
| Observational studies | |||||||||
| Total diet | Wosje et al. 2010 [335] | Prospective study with cross-sectional analysis by age to relate 3-day diet records to fat and bone mass in children during the age period of 3.8–7.8 years, using reduced-rank regression | Sex: male and female (167/158) Age: 3.8–7.8 years Race: 75 % white, 25 % black Location: Cincinnati, OH Year(s): 2000–2004 |
325 | Fat mass (kg) Bone mass (g) |
Diets high in dark-green and deep-yellow vegetables and processed meats and low in fried foods were associated with lower fat mass (P < 0.001) and higher bone mass (P = 0.03 for year 1, P = 0.2 for year 2, and P < 0.01 for years 3 and 4) | |||
| Fruits and Vegetables | Prynne et al. 2006 [189] | Cross-sectional Cambridge Bone Studies to relate fruit and vegetable and nutrient intake from 7-day food diaries in 5 age and sex cohorts | Sex: female and male, nearly half of each Age: 16–19 years Race: presumed white Location: UK |
257 | Percent change with doubling in fruit and vegetable intake from univariate analysis | ||||
| Boys | P | Girls | P | ||||||
| Total body BMC | 9.2 | <0.001 | 5.2 | 0.02 | |||||
| Spine BMC | 7.8 | 0.002 | 8.8 | 0.001 | |||||
| Total hip BMC | 6.6 | 0.008 | 5.0 | 0.04 | |||||
| Femoral neck BMC | 10.3 | <0.001 | 6.5 | 0.07 | |||||
| Trochanter BMC | 7.9 | 0.01 | 5.2 | NS | |||||
| Fruits and vegetables | McGartland et al. 2004 [195] | Cross-sectional study on effect of fruit and vegetable intake on BMD | Sex: male and female Age: 12 and 15 years Race: presumed white Location: Northern Ireland |
1345 | Forearm BMD Heel BMD |
12-year-old girls consuming high amounts of fruit had significantly higher heel BMD (β = 0.037; 95 % CI, 0.017, 0.056). No other associations were observed. | |||
| Fruits and vegetables | Tylavsky et al. 2004 [196] | Cross-sectional study on the effect of low (<3 servings) versus high (≥3 servings) fruit and vegetable intake on urinary calcium excretion and bone mass | Sex: female Age: 8–13 years Race: white Location: Tennessee, USA |
56 | Total body bone area Wrist bone area |
Compared with the low-consumption group, the high fruit and vegetable consumption group had 6 and 8.3 % larger total body (P < 0.03) and wrist bone area (P < 0.03). | |||
| Total body BMC Wrist BMC |
Whole body and wrist BMC was 7.4 (P = 0.07) and 7.0 % (P = 0.09) larger in the high-consumption group (P > 0.05). | ||||||||
| Total body BMD Wrist BMD |
Whole body and wrist BMD did not differ significantly between the low- and high-consumption groups (P > 0.05). | ||||||||
| Urinary calcium | Those reporting high fruit and vegetable intake had lower concentrations of urinary calcium/kg body weight (P < 0.02). | ||||||||
| Fruits and vegetables | Whiting et al. 2004 [197] | Cross-sectional study of bone growth in children | Sex: male and female Age: 8–14 years Race: presumed white Location: Saskatoon, Canada Year(s): 1991–1997 |
131 | BMC | Fruit and vegetable intake appears to influence BMC in adolescent girls but not boys. | |||
| Fruits and vegetables | Vatanparast et al. 2005 [198] | Cross-sectional study on the effect of milk products, vegetables, and fruit on total body BMC | Sex: male and female Age: 8–20 years Race: presumed white Location: Saskatoon, Canada Year(s): 1991–1997 |
150 | Total body BMC | Fruit and vegetable intake was a significant independent predictor of total body BMC in boys but not girls. | |||
| Caffeine | Conlisk and Galuska 2000 [204] | Cross-sectional study on effect of caffeine on BMD in healthy women | Sex: 177 women Age: 19–26 years Race: presumed white Location: Midwestern USA Year(s): 1991 |
177 | Caffeine consumption for past 12 weeks by self-report BMD at the lumbar spine and femoral neck by DXA |
Caffeine was not significantly associated with BMD. | |||
| Carbonated beverages | Wyshak 2000 [200] | Cross-sectional study of carbonated beverage consumption and bone fractures | Sex: girls Age: 9th and 10th graders (mean age 15.8 years) Race: unspecified, American high school students Location: “urban high school,” USA |
460 | Self-reported physical activity, carbonated beverage consumption, and bone fractures | Carbonated beverage consumption and bone fractures were associated (OR, 3.14; 95 % confidence limit, 1.45, 6.78; P = 0.004). | |||
| Carbonated beverages | McGartland et al. 2003 [194] | Cross-sectional observational study of the association between CSDs and BMD in postprimary schools in Northern Ireland | Sex: 744 girls, 591 boys Age: 12 years (323 boys, 376 girls); 15 years (268 boys, 368 girls) Race: presumed white Location: Belfast, Northern Ireland Year(s): 2000 |
1335 | CSD consumption via RD-administered dietary history method BMD of the nondominant forearm (distal radius) and dominant heel (os calcis) by DXA |
A significant inverse relationship between total CSD intake and BMD was observed in girls at the dominant heel (β, −0.099; 95 % CI, −0.173 to −0.025). Non-cola consumption was inversely associated with dominant heel BMD in girls (β, −0.121; 95 % CI, −0.194 to −0.048), and diet drinks were also inversely associated with heel BMD in girls (β, −0.087; 95 % CI, −0.158 to −0.016). No consistent relationships were observed between CSD intake and BMD in boys. | |||
| Carbonated beverages | Ma and Jones 2004 [199] | Population-based case–control study to investigate the association between soft drink and milk consumption, physical activity, bone mass, and upper limb fractures in children aged 9–16 years | Sex: half male and half female Age: 9–16 years Race: presumed white Location: Tasmania, Australia Year(s): 1998–2002 |
206 fractures 206 controls |
Bone mass using DXA at the total body, lumbar spine, right femoral neck: BMC aBMD BMAD Soft drink and dairy drink consumption (in-person interview) |
None of the drink types (milk, cola, and carbonated drinks) was significantly different between cases and controls for total fracture. For wrist and forearm fractures, there was a positive association between cola drink consumption and fracture risk (OR, 1.39/unit; 95 % CI, 1.01, 1.91). | |||
| Carbonated beverages | Manias et al. 2006 [201] | Cross-sectional study of recurrent fracture, diet, and physical activity | Sex: 78 girls, 72 boys Age: 4–16 years Race: presumed white Location: Sheffield, UK |
150 | Bone area, BMC, BMD of spine, lower body, and upper body by DXA Fracture history and trauma severity Diet (including beverage consumption), physical activity, and other lifestyle factors via questionnaires |
Children with recurrent fractures had a significantly lower milk intake, lower levels of physical activity, a higher BMI, and a higher consumption of carbonated beverages than controls. | |||
| Carbonated beverages | Libuda et al. 2008 [202] | Prospective (DONALD) study of diet from 3-day diet records for 4 years prior to a single forearm pQCT measure | Sex: 113 girls, 115 boys Age: 6–18 years Race: presumed white Location: Germany |
228 | Forearm pQCT | Carbonated beverage consumption was inversely associated with BMC (P < 0.05), cortical area (P < 0.05), and polar strength strain index (P < 0.05), polar strength strain index (P < 0.01), and periosteal circumference (P < 0.05) of the radius assessed by pQCT, after adjustment for age, sex, total energy intake, muscle area, BMI SD scores, and growth velocity. | |||
95 % CI 95 % confidence interval, aBMD areal bone mineral density, BMAD bone mineral apparent density, BMC bone mineral content, BMD bone mineral density, CSD carbonated soft drink, DONALD Dortmund Nutritional and Anthropometric Longitudinally Designed, DXA dual-energy x-ray absorptiometry, NS not significant, OR odds ratio, pQCT peripheral quantitative computed tomography, RCT randomized controlled trial, RD registered dietician, SSI stress–strain index
Table 9.
Infant nutrition and bone health in children and adolescents
| Source | Study description | Population description | Number of subjects | End points | Results | ||
|---|---|---|---|---|---|---|---|
| Koo et al. 2003 [211] | 6-month randomized, double-blind, parallel study assessing changes in bone mineral accretion by DXA in healthy infants fed a milk-based formula with or without palm olein | Sex: 57 males, 71 females Age: Infants >2 weeks at baseline Race:72 African American, 48 Caucasian, 8 Hispanic/Asian/other Location: USA and Canada |
128 | Total body BMC (%) | Group mean increase | ||
| Breast-fed | Control formula (milk-based formula) | Treatment (milk-based formula with palm olein) | |||||
| 3 months | 62.1 | 76.2* | |||||
| 6 months | 134.3 | 149.6* | |||||
| Observational studies | |||||||
| Butte et al. 2000 [216] | Prospective cohort study of breast-fed and formula-fed infants over 24 months | Sex: 33 males, 43 females Age: >2 weeks at baseline Race: 55 Caucasian, 7 African American, 11 Hispanic, 3 Asian Location: USA |
76 | Total body BMC (g) | Breast-fed (<12 months) | Breast-fed (>12 months) | Formula-fed |
| 12 months | Data not reported | Lowera | Highera,** | ||||
| 24 months | 310 (P = 0.05) | 289 (P = 0.05) | Data not reported | ||||
| Jones et al. 2000 [217] | Prospective cohort study to determine whether breastfeeding in early life is associated with bone mass in prepubertal children | Sex: 215 males, 115 females Age: 8 years at follow-up Race: predominantly Caucasian Location: Tasmania |
330 | Breast-fed | Bottle fed | ||
| Total body BMD at 8 years (g/cm2) | 0.642 ± 0.082 | 0.627 ± 0.073* | |||||
| Femoral neck BMD at 8 years (g/cm2) | 0.608 ± 0.072 | 0.590 ± 0.068 | |||||
| Lumbar spine BMD at 8 years, g/cm2 | 0.781 ± 0.047 | 0.766 ± 0.047** | |||||
| Ma and Jones 2003 [219] | Population-based case-controlled study to examine the association between bone mass and upper limb fractures in children | Sex: 206 males, 124 females Age: 8 years at follow-up Race: predominantly Caucasian Location: Tasmania |
324 | Breast-fed | |||
| Multivariate OR (95 % CI) | 0.43 (0.19–0.94)a | ||||||
| Young et al. 2005 [221] | BMD was assessed in healthy 4-year-old children after confirming the type of infant feeding by history. All children had exclusively consumed human milk, infant formula without palm olein oil, or an infant formula with palm olein oil. | Sex: 58 % male Mean age: 4.5 years Race: 85 % Caucasian Location: USA |
178 | Human milk | Formula without palm olein oil | Formula with palm olein oil | |
| Total body BMC at 4 years (g) | 566 ± 12 | 583 ± 10c | 570 ± 7 | ||||
| Harvey et al. 2009 [213] | Prospective cohort study examining associations between duration of breastfeeding and compliance with infant dietary guidelines and later bone size and density at age 4 years | Sex: 158 males, 149 females Age: 6 months at baseline Race: not reported Location: UK |
599 | Breast milk (<1 month) | Breast milk (2–6 months) | ||
| No differencea | No differencea | ||||||
| Molgaard et al. 2011 [212] | Random sample of infants from the Copenhagen Cohort Study of Infant Growth and Nutrition were investigated to determine if early nutrition and early growth are associated with later bone mass in adolescence. | Sex: 44 males, 65 females Age: birth to 12 weeks. Examination at 17 years Race: Danish origin; otherwise not reported Location: Denmark Years: 1987–2005 |
109 | Lumbar spine BMC | The duration of exclusive breastfeeding was positively correlated with the sex-adjusted lumbar spine BMCa,**. | ||
| Pirila et al. 2011 [214] | Prospective study of infants divided into three equal-sized groups according to the total duration of breastfeeding (short = 3 months, intermediate = >3 to <7 months, and prolonged = >7 months) followed-up after 32 years | Sex: 76 males, 82 females Age: 2 weeks to 12 months in original cohort; 31.7–34.0 years at follow-up Race: not reported Location: Finland Year(s): 2007–2009 |
158 | Total body BMC | In males, short breastfeeding was associated with higher bone area, BMC, and BMD compared to longer breastfeeding. Males in the short breastfeeding group had on average 4.7 % higher total body BMD than males in the prolonged breastfeeding groupa,**. | ||
| Fewtrell et al. 2013 [220] | To compare total body and lumbar spine bone in children aged 10 years that as infants participated in a randomized trial and either were breast-fed or randomly received a control formula or an sn-2 palmitate-enriched formula using a double-blind permuted block allocation. The study was completed at 12 weeks and follow-up measurements were taken at 10 years. | Sex: 52 % male, 48 % female Age: birth to 12 weeks; follow-up at 10 years Race: not reported Location: Cambridge, UK |
91 | Breast-fed | Formula with standard fat blend | Formula with high sn-2 fat blend | |
| Lumbar spine BMC (g) | 23.12 ± 4.46 | 22.54 ± 4.52 | 24.38 ± 5.15 | ||||
| Total body BMC (g) | 1062 ± 213 | 1049 ± 264 | 1097 ± 231 | ||||
| Jones et al. 2013 [218] | Birth cohort study to determine if early life factors (e.g., breastfeeding) were associated with bone mass and fractures in 16-year-old adolescents | Sex: 265 males, 150 females Age: 16 years at follow-up Race: predominantly Caucasian Location: Tasmania |
415 | OR (95 % CI) | Any fracture | Upper limb fracture | Lower limb fracture |
| Intention to breastfeed | 0.71 (0.55–0.94)c | 0.80 (0.55–1.66) | 0.63 (0.36–1.13) | ||||
| Breastfeeding at 1 month | 0.65 (0.48–0.87)c | 0.84 (0.54–1.73) | 0.49 (0.26–0.93)c | ||||
| Breastfeeding at 3 months | 0.80 (0.60–1.09) | 0.87 (0.58–1.31) | 0.82 (0.45–1.48) | ||||
| Maternal recall of breastfeeding | 0.69 (0.54–0.89)c | 0.73 (0.51–1.39) | 0.75 (0.42–1.34) | ||||
| Kalkwarf et al. 2013 [215] | A cross-sectional study to describe age, sex, race, growth, and human milk feeding effects on bone | Sex: 158 males, 149 females Age: 1–36 months Race: 225 Caucasian, 63 African American, 15 mixed Caucasian and African American, and 4 Asian Location: USA |
307 | Human milk | No human milk | ||
| Overall BMD Z-score | −0.05 | 0.21** | |||||
95 % CI confidence interval, BMC bone mineral content, BMD bone mineral density, DXA dual-energy x-ray absorptiometry, OR odds ratio, RCT randomized controlled trial
aValues were reported in a figure and via text
*P < 0.01; **P < 0.05
Table 10.
DMPA injections and oral contraceptive use on bone health in adolescents
| Source | Study description | Population description | Number of subjects | End points | Results | ||
|---|---|---|---|---|---|---|---|
| Observational studies | |||||||
| Lloyd et al. 2000 [223] | An 8-year longitudinal study of white females in the Penn State Young Women’s Health Study. OC users were those individuals who had used low-dose monophasic OCs for a minimum of 6 months at age ~12 and were still using them at age 20 years. | Sex: 62 females Age: 11.9 ± 0.5 years at baseline Race: Caucasian Location: USA |
62 | Total body BMC (g) at 12 years | OC users | Nonusers | |
| 1463.0 ± 64.0 | 1402.5 ± 45.5 | ||||||
| Total body BMC (g) at 20 years | 2272.2 ± 55.9 | 2214.2 ± 45.1 | |||||
| Total body BMD (g/cm2) at 12 years | 0.92 ± 0.01 | 0.91 ± 0.01 | |||||
| Total body BMD (g/cm2) at 20 years | 1.13 ± 0.01 | 1.12 ± 0.01 | |||||
| Hip BMD (g/cm2) at 20 years | 1.01 ± 0.02 | 0.99 ± 0.02 | |||||
| The OC users did not differ in anthropometric, body, or total bone measurements at baseline or at age 20 years (no differences in age at menarche or exercise at age 20 years). OC pill use by healthy white teenage females did not affect acquisition of peak bone mass. |
|||||||
| Lara-Torre et al. 2004 [225] | Nonrandomized prospective study to examine BMD in control adolescent subjects vs those receiving DMPA injections or OCs over a 2-year period | Sex: 71 females Age: 11–19 years Race: Caucasian and black Location: USA |
148 | OC usersb | DMPA injectionb | Nonuserb | |
| % change in lumbar BMD at 6 months | 1.170 (−0.14, 2.48) | −0.249 (−1.25, −0.75)* | 2.768 (0.48, 5.05) | ||||
| % change in lumbar BMD at 12 months | 2.35 (0.16, 3.78) | −1.59 (−2.53, 0.59)* | 2.45 (−2.01, 5.99) | ||||
| % change in lumbar BMD at 18 months | 3.82 (−0.62, 6.41) | −2.91 (−3.64, −2.23)* | 0.73 (−1.01, 1.54) | ||||
| % change in lumbar BMD at 24 months | −1.01 (−1.23, 5.33) | −1.85 (−4.15, −0.23)* | 5.89 (5.37, 6.85) | ||||
| There was a statistically significant difference in BMD between DMPA users and the control at 6, 12, 18, and 24 months. There was no statistical difference between OC pill users and the control at any time point. | |||||||
| Lloyd et al. 2004 [224] | A 10-year longitudinal study of white females in the Penn State Young Women’s Health Study. OC users were those individuals who had used low-dose monophasic OCs for a minimum of 6 months at age ~12 years and were still using them at age 22 years. | Sex: 80 females Age: 21.7 ± 0.1 years Race: Caucasian Location: USA |
80 | OC users | Nonusers | ||
| Total body BMC (g) at 22 years | 2260 ± 55 | 2316 ± 66 | |||||
| Total body BMD (g/cm2) at 22 years | 1.14 ± 0.01 | 1.15 ± 0.02 | |||||
| Hip BMD (g/cm2) at 22 years | 1.0 ± 0.02 | 1.0 ± 0.02 | |||||
| Femoral neck section modulus (cm3) at 22 years | 1.29 ± 0.05 | 1.32 ± 0.05 | |||||
| Femoral neck section modulus (cm3) at 22 years | 1.70 ± 0.06 | 1.74 ± 0.07 | |||||
| OC pill use among adolescents was not correlated with bone or body composition measurements. | |||||||
| Rome et al. 2004 [227] | Prospective, observational design study to examine the effects of DMPA injections or OC (20 μg ethinyl estradiol/100 μg levonorgestrel) use on bone biochemical markers over 12 months | Sex: 165 females Age: 11–18 years Race: Caucasian and black Location: USA |
370 | Spine and femoral neck BMD | No significant differences reported between spine and femoral neck BMD for each group at each time point. Spine BMD was lower in the DMPA injection group vs the OC group. Exact values not reported | ||
| Cromer et al. 2008 [226] | Observational prospective cohort study to examine the effects of DMPA injections or OC (20 μg ethinyl estradiol/100 μg levonorgestrel) use on BMD over 24 months | Sex: 375 females Age: 12–18 years Race: Caucasian and black Location: USA |
433 | OC users | DMPA injection | Nonuser | |
| Spine BMD (g/cm2) | 1.03 ± 0.11 | 0.98 ± 0.09* | 0.98 ± 0.11 | ||||
| Femoral neck BMD (g/cm3) | 0.97 ± 0.14 | 0.92 ± 0.14* | 0.92 ± 0.15 | ||||
| Over 24 months, mean percent change in spine BMD was −1.5 % (DMPA injection), 4.2 % (OC use), and 6.3 % (control). Over 24 months, mean percent change in femoral neck BMD was −5.2 % (DMPA injection), 3.0 % (OC use), and 3.8 % (control). Adolescent girls receiving DMPA injections had significant loss of BMD. Clinical significance of this loss may be mitigated by the slowed loss after the first year of DMPA use. |
|||||||
| Pikkarainen et al. 2008 [228] | 4-year follow-up study examining the effects of estrogen-progestin OC use | Sex: 122 females Age: 12–19 years Race: Assumed predominantly Caucasian Location: Finland |
122 | OC user (1–2 years) | OC user (>2 years) | Nonuser | |
| Lumbar spine area (cm2) at baseline | 56.1 ± 6.1 | 56.7 ± 5.9 | 55.1 ± 5.9 | ||||
| Lumbar spine area (cm2) at 4 years | 58.6 ± 6.1 | 58.7 ± 0.7 | 59.5 ± 6.3 | ||||
| Femoral neck area (cm2) at baseline | 4.79 ± 4.33 | 4.64 ± 0.37 | 4.66 ± 0.40 | ||||
| Femoral neck area (cm2) at 4 years | 4.80 ± 0.3 | 4.7 ± 0.4 | 4.7 ± 0.40 | ||||
| Lumbar spine BMC (g) at baseline | 54.3 ± 11.8 | 57.5 ± 11.9 | 51.2 ± 10.2 | ||||
| Lumbar spine BMC (g) at 4 years | 59.10 ± 11.7 | 60.2 ± 12.3 | 59.2 ± 10.0 | ||||
| Femoral neck BMC (g) at baseline | 4.3 ± 0.8 | 4.4 ± 0.8 | 4.1 ± 0.6 | ||||
| Femoral neck BMC (g) at 4 years | 4.5 ± 0.8 | 4.4 ± 0.7 | 4.3 ± 0.6 | ||||
| There was a significant trend of a lesser increase of BMC in lumbar spine in the OC users (>2 years) than in the other two groups (P < 0.0046). The development of the femoral neck was significantly different between the OC users (1–2 years) and OC users (>2 years). The longer duration of OC use seemed to suppress normal BMC development (P for linearity = 0.038) |
|||||||
| Walsh et al. 2008 [230] | A case-controlled matched study aiming to examine whether the effects of DMPA injections are age-specific and to determine the effects of DMPA on hormones and bone turnover | Sex: 100 females (50 pairs) Age: 12–18 years Race: Caucasian and black Location: USA |
100 | DMPA injectiona | Nonusersa | ||
| Lumbar spine BMD (g/cm2) | 1.003 (0.972–1.035)* | 0.947 (0.950–0.977) | |||||
| Total hip BMD (g/cm2) | 0.983 (0.950–1.016)* | 0.931 (0.897–0.966) | |||||
| Distal forearm BMD (g/cm2) | 0.465 (0.446–0.483) | 0.474 (0.457–0.491) | |||||
| DMPA injections are associated with a bone density deficit at the spine and hip when used before peak bone mass. | |||||||
| Bonny et al. 2011 [222] | Prospective longitudinal study examining the relationship between weight and BMD and the correlation between weight change and BMD in premenarcheal girls reporting DMPA use or OC (20 μg ethinyl estradiol/100 μg levonorgestrel) use | Sex: 433 females Age: 12–18 years Race: Caucasian and black Location: USA |
433 | User | DMPA injection | Nonuser | |
| Correlation between absolute change in weight (kg) and absolute change in femoral neck BMD at 12 months | 1.000 | 1.000 | 1.000 | ||||
| Correlation between absolute change in weight (kg) and absolute change in femoral neck BMD at 24 months | 1.000 | 1.000 | 1.000 | ||||
| Correlation between absolute change in weight (kg) and absolute change in spine BMD at 12 months | −0.82 | −0.101 | 0.10 | ||||
| Correlation between absolute change in weight (kg) and absolute change in spine BMD at 24 months | −0.12 | 0.098 | 0.48 | ||||
| Body weight was significantly positively associated with femoral neck BMD and spine BMD regardless of the contraceptive method (P < 0.05). Change in body weight at 12 and 24 months was highly correlated with change in femoral neck BMD (P < 0.0001) for all treatment groups. No statistically significant correlation between change in weight and change in spine BMD was seen in the DMPA, OC, or control subjects at 12 or 24 months. |
|||||||
| Biason et al. 2015 [229] | Nonrandomized parallel-control study with 1-year follow-up assessing the effect of OC (20 μg ethinyl estradiol/150 μg desogestrel) users on bone density | Sex: 67 females Age: 12–19 years Race: Assumed Brazilian Location: Brazil |
67 | OC user | Nonuser | ||
| % variation lumbar BMD (g/cm2) | 2.07 | 12.16 | |||||
| % variation lumbar BMC (g) | 1.57* | 16.84 | |||||
| % variation subtotal BMD (g/cm2) | 0.56* | 5.28 | |||||
| % variation subtotal BMC (g) | 1.18* | 16.04 | |||||
| % variation whole body BMD (g/cm2) | 0.84 | 5.28 | |||||
| % variation whole body BMC (g) | 1.22* | 11.34 | |||||
| Use of low-dose OCs was associated with lower bone mass acquisition in adolescents over a 1-year period. OC users showed low bone mass acquisition in the lumbar spine and had BMD and BMC median variations of 2.07 and 1.57 %, respectively, between the measurements at baseline and 12 months. Nonusers showed median variations of 12.16 and 16.84 % for BMD and BMC, respectively, over the same period. |
|||||||
95 % CI confidence interval, BMC, bone mineral content, BMD bone mineral density, RCT randomized controlled trial
aMean (95 % CI)
bMedian (25 %, 75 %)
*P < 0.05 compared with control
Table 11.
Alcohol consumption and bone health in children and adolescents
| Reference | Study description | Population description | Number of subjects | End points | Results | |||
|---|---|---|---|---|---|---|---|---|
| Prospective studies | ||||||||
| Korkor et al. 2009 [231] | This 4-year prospective study assessed the relationship between smoking and alcohol intake and bone mass. Students in 9th grade were recruited. Number of alcoholic beverages in the last week was reported in the 9th through 12th grades. Subjects classified as any alcohol over 4 years vs not | Sex: 37 males, 72 females Age: 14–19 years at enrollment Race: white 83 %, Asian 1 %, Hispanic 4 %, unknown 12 % Location: Wisconsin, USA Year(s): 2000–2003 |
109 | Peripheral DXA | Simple model, any alcohol intake | Adjusted model, any alcohol, or smoking | ||
| Heel aBMD in 12th grade (g/cm2) | −0.042, P = 0.047 (−7.2 %) | −0.028, P = 0.05 (−4.8 %) | ||||||
| The simple model includes any alcohol over the 4-year study (yes, n = 13; no, n = 96). The adjusted model was adjusted for sex baseline aBMD dairy intake. Large overlap in smoking and alcohol use. Any smoking or alcohol combined (N = 14) was associated with a −0.028-g/cm2 lower aBMD. | ||||||||
| Lucas et al. 2012 [235] | This prospective study quantified the association between early initiation of smoking and alcohol intake and forearm BMD in early and late adolescence. Alcohol intake reported at age 13 and 17 years and classified as never, tried but not currently drinking, <1 drink/week, or ≥1 drink/week | Sex: female Age: 13 and 17 years Race: not specified Location: Portugal Year(s): 2003–2007 |
716 | Peripheral DXA | Drinking at age 13 years vs never (n = 298) | |||
| Distal radius aBMD | Tried, not currently drinking (n = 380) | Drinks < 1/week (n = 26) | Drinks ≥ 1/week (n = 8) | P value | ||||
| At age 13 years | 0.3 % | −2.0 % | −1.1 % | 0.826 | ||||
| At age 17 years | −1.1 % | 2.3 % | −4.8 % | 0.282 | ||||
| Drinking at age 17 years vs never (n = 120) | ||||||||
| Tried, not currently(n = 273) | Drinks, not daily (n = 266) | Drinks, daily (n = 54) | P value | |||||
| At age 17 years | 1.1 % | 0.0 % | −2.3 % | 0.471 | ||||
| Ever drank in adolescence vs never (n = 84) | ||||||||
| Tried 13 years but before 17 years (n = 213) | Tried before 13 years (n = 419) | P value | ||||||
| −0.9 % | −2.0 % | 0.216 | ||||||
| Means are adjusted for menarche age, alcohol intake, sports, and BMI. | ||||||||
| Dorn et al. 2013 [233] | This 3-year prospective study of girls examined bone accrual according to smoking, alcohol intake, depression, and anxiety. Alcohol use was coded as 0–5 drinks or ≥6 drinks in lifetime. | Sex: female Age: 11–17 years Race: 62 % white, 33 % black, 5 % other Location: Ohio, USA Year(s): 2003–2010 |
262 | DXA Total body BMC (g) Spine aBMD (g/cm2) Hip aBMD (g/cm2) |
There was no significant association between alcohol use and any bone outcome measure. Regression models were adjusted for race, puberty stage, weight, height, age at menarche, contraceptive use, calcium intake, vitamin D status, and physical activity. | |||
| Cross-sectional studies | ||||||||
| Elgan et al. 2002 [237] | This cross-sectional study conducted in nursing students measured lifestyle and physiologic factors. Alcohol intake was measured by questionnaire. | Sex: female Age: 16–24 years Race: not reported Location: Lund, Sweden Year(s): 1999 |
218 | Peripheral DXA Heel aBMD (g/cm2) |
Correlation with alcohol intake, r = −0.05 (P = NS) Alcohol intake not significant (P = 0.84) in regression models adjusting for age, weight, physical activity, and hormonal age Average alcohol intake 16.6 g/month; 79 % reported some alcohol intake |
|||
| Kyriazopoulos et al. 2006 [236] | This cross-sectional study evaluated the influence of current dietary factors (calcium, proteins, alcohol, coffee, and tea intake), exercise, smoking, and sunlight on forearm bone mass in young Greek men. Alcohol intake reported as consumption per week | Sex: male Age: 18–30 years, mean 22 years Race: not reported Location: Greece Year(s): not reported |
300 | Peripheral DXA Distal radius BMC (g) Distal radius aBMD (g/cm2) Ultradistal radius aBMD (g/cm2) |
Mean alcohol intake 3.78 ± 1.35 times/week. No association between alcohol intake (times/week) and bone measures with and without adjustment for height, weight, calcium intake, sunlight exposure, exercise, and work | |||
| Dorn et al. 2011 [232] | This cross-sectional analysis of baseline data examined how bone mass and density varied according to smoking, alcohol intake, depression, and anxiety. Alcohol intake reported as no drinks (n = 135), 1–5 drinks (n = 59), ≥6 drinks (n = 67) in the past year | Sex: female Age: 11–17 years Race: 62 % white, 33 % black, 5 % other Location: Ohio, USA Year(s): 2003–2007 |
261 | DXA Total body BMC (g) Spine aBMD (g/cm2) Total hip aBMD (g/cm2) Femoral neck aBMD (g/cm2) |
No significant main effect of alcohol group and any outcome measure (P > 0.10). Analyses were adjusted for age, weight, height, race, and maturational stage. | |||
| Significant interactions between alcohol, smoking, and depression symptoms on bone outcomes. Stronger negative association between depressive symptoms and total body BMC among individuals who smoked and used alcohol | ||||||||
| Eleftheriou et al. 2013 [238] | A cross-sectional study evaluating the association of smoking, alcohol consumption, and prior exercise with lower limb bone volume, composition, and structure by MRI and DXA in a large cohort of healthy Caucasian males. Alcohol intake was coded as none, low (1–9 U/week), moderate (12–21 U/week), or high (>21 U/week) | Sex: male Age: mean 19.9 years Race: Caucasian Location: UK Year(s): not reported |
651 | DXA | Difference between Alcohol intake group vs none | |||
| Low 1–9 U/week | Moderate 12–24 U/week | High > 24 U/week | P value | |||||
| Total hip aBMD | 1.5 % | 2.3 % | −0.6 % | 0.017 | ||||
| Femoral neck aBMD | −1.1 % | 2.8 % | −0.7 % | 0.015 | ||||
| Proximal femur geometry by MRI | ||||||||
| Periosteal volume | NS, data not shown | |||||||
| Endosteal volume | NS, data not shown | |||||||
| Cortical volume | NS, data not shown | |||||||
| Adjusted for height, weight, smoking, and weight-bearing activity | ||||||||
| Winther et al. 2014 [234] | This cross-sectional, population-based study compared BMD levels of Norwegian adolescents with lifestyle factors. Alcohol intake reported as never, ≤1 per month, or ≥2 per month. Alcohol use recoded for analysis as yes vs no | Sex: male and female Age: 15–17 years Race: not reported Location: Norway Year(s): 2010–2011 |
835 | Difference between alcohol users vs not | ||||
| Age adjusted | Multivariable adjusted | |||||||
| DXA | β | P value | β | P value | ||||
| Males (n = 492) | ||||||||
| Total hip aBMD (g/cm2) | 0.027 | 0.057 | 0.035 | 0.006 | ||||
| Femoral neck aBMD (g/cm2) | 0.030 | 0.037 | 0.028 | 0.021 | ||||
| Females (n = 469) | ||||||||
| Total hip aBMD (g/cm2) | −0.014 | 0.297 | – | – | ||||
| Femoral neck aBMD (g/cm2) | −0.013 | 0.330 | – | – | ||||
| Alcohol uses include intakes of up to 1/month (44.7 % of girls, 36.4 % of boys) and ≥2 times/month (30.3 % of girls, 30.9 % of boys) vs never (23.4 % of girls, 30.4 % of boys). Multivariable analyses were adjusted for age, BMI, height, sexual maturation, physical activity, smoking, diseases, and medications known to affect bone hormonal contraceptives. |
||||||||
aBMD areal bone mineral density, BMC bone mineral content, BMD bone mineral density, DXA dual-energy x-ray absorptiometry, NS not significant
Table 12.
Smoking and bone health in children and adolescents
| Reference | Study description | Population description | Number of subjects | End points | Results | |||
|---|---|---|---|---|---|---|---|---|
| Prospective studies | ||||||||
| Lappe et al. 2001 [42] | This prospective study of female army recruits examined risk factors for developing stress fractures during 8 weeks of basic training. | Sex: female Age: 21.1 ± 3.7 years Race: 31 % black, 53 % white, 16 % other Location: USA Years: 1995–1996 |
3758 | Stress fracture | Odds ratios (95 % CI) | |||
| History of smoking 1.34 (1.05–1.71) Years smoked 1.05 (1.02–1.08) | ||||||||
| •Adjusted for age, race and bone speed of sound | ||||||||
| Elgan et al. 2003 [240] | This prospective study is a 2-year follow-up of the cohort reported in 2002 and investigated the joint effects of OC use and smoking. Smoking reported as monthly average. Twenty-eight subjects smoked as follows: n = 1, >25 cig/day; n = 6, 15–24 cig/day; n = 16, 5–14 cig/day;, n = 4, ≤4 cig/day. | Sex: female Age: 18–26 Race: not reported Location: Lund, Sweden Year(s): 2001 |
118 | Peripheral DXA | Difference from reference group, nonsmoker/no OC use (n = 35) | |||
| Heel aBMD | Smoker/no OC use (n = 9) | Nonsmoker/OC use (n = 57) | Smoker/OC use (n = 17) | |||||
| T2 aBMD (g/cm2) | ||||||||
| Unadjusted | 0.0013 | 0.014 | −0.012 | |||||
| Adjusteda | −0.032* | −0.014 | −0.017* | |||||
| 2-year change in aBMD (g/cm2) | ||||||||
| Unadjusted | −0.035* | −0.013 | −0.016 | |||||
| Adjusteda | −0.06 | −0.010 | −0.021 | |||||
|
aAdjusted for age, physical activity baseline BMD, body weight *P < 0.05 | ||||||||
| Lappe et al. 2008 [241] | Secondary analyses of this randomized calcium and vitamin D supplementation trial examined other risk factors for developing stress fractures during basic training. | Sex: female Age: 19 years (17–35 years) Race: not specified Location: USA Years 2001–2006 |
5201 | Stress fracture | Risk of fracture was 41 % higher in women with history of smoking when adjusted for treatment group, P = 0.0075 Odds ratio for fracture was 1.32 (0.99–1.75) in women with history of smoking when adjusted for treatment, amenorrhea, high exercise, Depo Provera use, age, and running speed. |
|||
| Korkor et al. 2009 [231] | This 4-year prospective study assessed the relationship between smoking and alcohol intake and bone mass. Students in 9th grade were recruited. Number of cig smoked per day reported in the 9th, 10th, 11th, and 12th grades. Subjects classified as any smoking over 4 years vs not | Sex: 37 males, 72 females Age: 14–19 years at enrollment Race: white 83 %, Asian 1 %, Hispanic 4 %, unknown 12 % Location: Wisconsin, USA Year(s): 2000–2003 |
109 | Peripheral DXA | Simple model, any smokinga | Adjusted model, any alcohol or smokingb | ||
| Heel aBMD in 12th grade (g/cm2) | −0.0249, P = 0.29 | −0.0281, P = 0.05 | ||||||
| −4.3 % | −4.8 % | |||||||
|
aAny smoking over the 4-year study (yes, n = 8; no, n = 101) bAdjusted for sex baseline aBMD dairy intake Large overlap in smoking and alcohol use. Any smoking or alcohol combined (N = 14) was associated with a −0.028-g/cm2 lower aBMD. | ||||||||
| Lucas et al. 2012 [235] | This prospective study quantified the association between early initiation of smoking and alcohol intake and forearm BMD in early and late adolescence. Smoking reported at age 13 and 17 years and classified as: tried but not currently smoking, smokes but not daily or smokes daily | Sex: female Age: 13 years and 17 years Race: not specified Location: Portugal Year(s): 2003–2007 |
713 | Peripheral DXA | Smoking at age 13 years vs never smoked (n = 523) | |||
| Distal radius aBMD | Tried, not currently smoking (n = 165) | Smokes (n = 24) | P value | |||||
| At age 13 years | 1.1 % | −0.6 % | 0.410 | |||||
| At age 17 years | −2.3 % | −0.7 % | 0.068 | |||||
| Smoking at age 17 years vs never (n = 390) | ||||||||
| Tried, not currently (n = 224) | Smokes, not daily (n = 33) | Smokes, daily (n = 72) | P value | |||||
| At age 17 years | −2.3 % | −3.2 % | −0.7 % | 0.438 | ||||
| Ever smoked in adolescence vs never smoked (n = 353) | ||||||||
| At age 17 years | Tried 13 years but before 17 years (n = 167) | Tried before 13 years (n = 192) | P value | |||||
| 0.4 % | −1.8 % | 0.103 | ||||||
| Adjusted for menarche age, alcohol intake, sports, and BMI | ||||||||
| Dorn et al. 2013 [233] | This 3-year prospective study of girls examined bone accrual according to smoking, alcohol intake, depression, and anxiety. Smoking measured every 3 months and coded as cigarettes smoked in last 30 days: 0, 1–2 days, 3–5 days, 6–9, 10–19 days, 20–29 days, or 30 day | Sex: female Age: 11–17 years Race: 62 % white, 33 % black, 5 % other Location: Ohio, USA Year(s): 2003–2010 |
262 | DXA Total body BMC (g) Spine aBMD (g/cm2) Hip aBMD (g/cm2) |
No significant main effect or age × smoking interaction on total body Significant age × smoking interaction for spine and hip aBMD. As smoking increased, the rate of bone accrual decreased as girls got older. Regression models adjusted for race, puberty stage, weight, height, and contraceptive use |
|||
| Cross-sectional studies | ||||||||
| Elgan et al. 2002 [237] | This cross-sectional study conducted in nursing students measured lifestyle and physiologic factors. Smoking was measured by questionnaire and dichotomized as yes/no. 23 % smoked on a daily basis, 22 % were party smokers, and 55 % were nonsmokers. | Sex: female Age: 16–24 years Race: not reported Location: Lund, Sweden Year(s): 1999 |
218 | Peripheral DXA Heel aBMD (g/cm2) |
Smoking status not significantly associated with aBMD in bivariate analyses. Data not shown Smoking not significant (P = 0.48) in regression models adjusting for age, weight, physical activity, and hormonal age |
|||
| Afghani et al. 2003 [336] | A cross-sectional analysis of baseline data from a smoking cessation trial. Analyses investigated the role of body composition, physical activity, menarche, smoking, and second-hand smoke on regional bone mass among Asian adolescents living in Wuhan, China. Smoking questions assessed if subjects “smoked at least 100 cigarettes in their life?” and “have smoked in last 30 day.” Smoking was reclassified for analyses as smoking yes/no. | Sex: male and female Age: 12–16 years, 14.5 ± 0.5 Race: Asian Location: Wuhan, China Year(s): not reported |
466 | Peripheral DXA | Correlation with smoking (yes/no)a | P value | Multivariable modelsb | |
| Boys (n = 300) | ||||||||
| Forearm BMC | 0.05 | NS | NS | |||||
| Forearm aBMD | 0.04 | NS | – | |||||
| Heel BMC | 0.05 | NS | NS | |||||
| Heel aBMD | 0.00 | NS | – | |||||
| Girls (n = 166) | ||||||||
| Forearm BMC | 0.15 | <0.05 | NS | |||||
| Forearm aBMD | 0.04 | NS | – | |||||
| Heel BMC | 0.08 | NS | NS | |||||
| Heel aBMD | 0.09 | NS | – | |||||
|
aUnclear how correlation derived given that smoking was a dichotomous variable bAdjusting for age, height, lean mass, fat mass, team sports participation, menarche, and passive smoking Low level of smoking and inconsistent report of smoking in the sample limits conclusions. 38 % of girls and 14 % of boys inconsistently answered smoking questions. Among smokers, 58 % of girls and 51 % of boys smoked ≤1 cigarette in last 30 days. | ||||||||
| Kyriazopoulos et al. 2006 [236] | This cross-sectional study evaluated the influence of current dietary factors (calcium, proteins, alcohol, coffee, and tea intake), exercise, smoking, and sunlight on forearm bone mass in young Greek men. Smoking coded as daily smoking (58.6 %) or no | Sex: male Age: 18–30 years, mean 22 years Race: not reported Location: Greece Year(s): not reported |
300 | Peripheral DXA Distal Radius BMC Distal radius aBMD Ultradistal radius aBMD |
There was no association between daily smoking (58.6 % of sample) and bone measures with and without adjustment for height, weight, calcium intake, sunlight exposure, exercise, and work. Data not shown | |||
| Lorentzon et al. 2007 [239] | The GOOD study is a cross-sectional study involving a random selection of males in Gothenburg. Bone mass, density, and geometry measured by DXA and pQCT. Smoking quantified as cigarettes/day and duration. For analyses, subjects were classified as smokers ≥1 cig/day vs not. | Sex: male Age: 18–20 years Race: not reported Location: Gothenburg, Sweden Year(s): not reported |
1063 | Difference between smoked ≥1 cigarette/daya vs not | ||||
| Unadjusted | P value | Adjustedb | P value | |||||
| DXA | ||||||||
| Total body aBMD | −2.1 % | <0.01 | −1.8 % | 0.01 | ||||
| Spine aBMD | −4.3 % | <0.001 | −3.3 % | <0.01 | ||||
| Femoral neck aBMD | −5.3 % | <0.001 | −3.9 % | <0.01 | ||||
| Trochanter aBMD | −6.6 % | <0.001 | −5.0 % | <0.01 | ||||
| pQCT | ||||||||
| Tibia | ||||||||
| Cortical vBMD | 0.0 % | 0.91 | – | NS | ||||
| Cortical thickness | −4.5 % | <0.001 | −4.0 % | <0.001 | ||||
| Periosteal circumference | 0.0 % | 0.97 | – | NS | ||||
| Endosteal circumference | 2.5 % | <0.05 | 2.7 % | 0.01 | ||||
| Trabecular vBMD | −4.2 % | <0.01 | −3.8 % | <0.01 | ||||
| Radius | ||||||||
| Cortical vBMD | −0.1 % | 0.55 | – | NS | ||||
| Cortical thickness | −2.8 % | <0.01 | −2.9 % | <0.01 | ||||
| Periosteal circumference | 0.7 % | 0.32 | – | NS | ||||
| Endosteal circumference | 3.3 % | 0.02 | 4.5 % | <0.01 | ||||
| Trabecular vBMD | −2.7 % | 0.16 | – | NS | ||||
|
aSmokers consumed 9.3 ± 6.3 cig/day. Mean duration of smoking 4.1 ± 2.1 years. Smokers less active than nonsmokers bAdjusted for calcium intake, physical activity, age, height, and weight. Smokers had reduced BMD due to smaller cortical thickness greater endosteal circumference | ||||||||
| Dorn et al. 2011 [232] | This cross-sectional analysis of data examined how bone mass and density varied according to smoking, alcohol intake, depression, and anxiety. Smoking ever in their life coded as never (n = 104), 1 puff to 2 cig (n = 54), and 3–99 cig (n = 51), >100 cig/day (n = 52) | Sex: female Age: 11–17 years Race: 62 % white 33 % black, 5 % other Location: Ohio, USA Year(s): 2003–2007 |
261 | DXA | ||||
| Total body BMC (g) | No significant differences among smoking groups | |||||||
| Spine aBMD (g/cm2) | No significant differences among smoking groups | |||||||
| Total hip aBMD (g/cm2) | 1 puff to 2 cig group 6.5 % greater than >100 cig group, P < 0.05 | |||||||
| Femoral neck aBMD (g/cm2) | 1 puff to 2 cig group 6.0 % greater than >100 cig group, P < 0.05 | |||||||
| Analyses adjusted for age, weight, height, race, and maturational stage. Significant interactions between alcohol and tobacco use, and depression symptoms on bone outcomes. Stronger negative association between depressive symptoms and total body BMC among individuals who smoked and used alcohol. | ||||||||
| Eleftheriou et al. 2013 [238] | A cross-sectional study evaluating the association of smoking, alcohol consumption, and prior exercise with lower limb bone volume, composition, and structure by MRI and DXA in a large cohort of healthy Caucasian males. Smoking status coded as nonsmoker, ex-smoker (>6 months), recent ex-smoker (≤6 months), or current smoker | Sex: male Age: mean 19.9 years Race: Caucasian Location: UK Year(s): not reported |
651 | Differencea between smoking status vs nonsmoker (n = 329) | ||||
| Ex-smoker >6 months (n = 41) | Recent ex-smoker ≤ 6 months (n = 35) | Current smoker (n = 244) | P value | |||||
| DXA | ||||||||
| Total hip aBMD | −5.0 % | −6.0 % | −4.7 % | 0.0001 | ||||
| Femoral neck aBMD | −5.9 % | −5.3 % | −4.0 % | 0.001 | ||||
| MRI | ||||||||
| Periosteal volume | −5.0 % | −4.3 % | −0.4 % | 0.004 | ||||
| Endosteal volume | NS, data not shown | |||||||
| Cortical volume | NS, data not shown | |||||||
| aAdjusted for height weight, alcohol intake, and weight-bearing activity | ||||||||
| Winther et al. 2014 [234] | This cross-sectional population-based study compared BMD levels of Norwegian adolescents with lifestyle factors. Smoking was classified as daily smoking, sometimes smokes, or never smokes. | Sex: male and female Age: 15–17 years Race: Location: Norway Year(s): 2010–2011 |
835 | Difference between smokinga vs not | ||||
| Age adjusted | Multivariable adjusteda | |||||||
| DXA | Beta | P value | Beta | P value | ||||
| Males (n = 492) | ||||||||
| Total hip aBMD (g/cm2) | −0.039 | 0.012 | 0.029 | 0.037 | ||||
| Femoral neck aBMD (g/cm2) | −0.025 | 0.116 | – | – | ||||
| Females: (n = 469) | ||||||||
| Total hip aBMD (g/cm2) | −0.026 | 0.074 | – | – | ||||
| Femoral neck aBMD (g/cm2) | −0.031 | 0.031 | – | – | ||||
|
aIncludes daily smoking (5.5 % of girls and 3.8 % of boys) and sometimes smokes (15.9 % girls, 20.2 % of boys) vs never smokes (76.6 % of girls and 74.2 % of boys) bAdjusted for age, BMI, height, sexual maturation, physical activity, alcohol intake, diseases, and medications known to affect bone and hormonal contraceptives | ||||||||
aBMD areal bone mineral density, BMC bone mineral content, BMD bone mineral density, cig cigarette, DXA dual-energy x-ray absorptiometry, GOOD Gothenburg osteoporosis and obesity determinants, OC oral contraceptive, pQCT peripheral quantitative computed tomography
Table 13.
Physical activity and exercise on bone mass and density in children and adolescents
| Reference | Study description | Population description | Number of subjects | Intervention | End points | Results | |||
|---|---|---|---|---|---|---|---|---|---|
| RCTs | |||||||||
| Witzke and Snow 2000 [337] | 9-month jumping intervention Recruited for exercise group No randomization |
Sex: female Age: 13–15 years Race: not listed Location: Corvallis, OR |
53 | Frequency: 3×/week Intensity: varied Time: 30–45 min/session Type: resistance training: squats, lunges, toe/heel raises, bench stepping, and jumping. Plyometric training: two-footed, in-place jumps to stair jumping, bounding, depth jumps Control: regular PE |
DXA | Percent change, mean ± SD | |||
| Significant | EX | CON | |||||||
| TR BMC | 3.13 ± 6.44* | 1.96 ± 6.69 | |||||||
| NS | Both groups experienced a significant increase in percent change in bone mass compared to zero for TB, FN, LS, and FS, but only EX improved BMC of the TR (3.1 vs 1.9 %). | ||||||||
| TB, FN, LS, FS BMC | |||||||||
| McKay et al. 2000 [338] | 8-month loading intervention Randomized by school |
Sex: male and female Age: 6.9–10.2 years Race: 34 % Asian, 66 % white Location: Richmond, British Columbia |
144 | Frequency: 3×/week (2× PE, 1× classroom) Intensity: progressive Time: 10–30 min (minimum 10 min loading) Type: variety: games, circuit training, dances. Ten tuck jumps required at beginning of each session Control: regular PE |
DXA | Percent change, mean ± SEM | |||
| Significant | EX | CON | |||||||
| TR aBMD | 4.4 ± 0.5* | 3.2 ± 0.3 | |||||||
| NS | EX group showed significantly greater change in TR aBMD than CON group (4.4 vs 3.2 %). No group differences at other sites. | ||||||||
| TB, LS, PF, FN aBMD | |||||||||
| Heinonen et al. 2000 [244] | 9-month step aerobics/jumping intervention Randomized by school |
Sex: female Age: 10–15 years Race: not specified Location: Tampere, Finland |
126 | Frequency: 2×/week Intensity: progressive Time: 50 min (10-min warm-up, 15-min nonimpact aerobic exercises, 20-min high-impact/jump training, 5-min cool down) Type: both-leg jumps at floor level, both-leg box jumps, one-leg box jumps Control: normal PA |
DXA | Difference (g), mean (95 % CI) | |||
| Significant | Premenarchal EX vs CON |
Postmenarchal EX vs CON |
P value Effect of EX (pre vs post) |
||||||
| LS BMC | 1.01 (0.23, 1.78)* | ||||||||
| FN BMC | 0.09 (0.02, 0.15)* | * | |||||||
| TR BMC | * | ||||||||
| In the premenarchal girls, BMC increased significantly more in the EX than in CON at LS (8.6 vs 5.3 %) and FN (9.3 vs 5.3 %). The postmenarchal girls showed no significant posttraining intergroup differences at any site. | |||||||||
| Fuchs et al. 2001 [306] | 7-month jumping intervention Randomized by gender |
Sex: male and female Age: 7.5 years ± 0.17 Race: 87 white, 1 Asian, 1 white-Hispanic Location: Corvallis, OR |
89 | Frequency: 3×/week, opposite PE Intensity: progressed from 50 to 80 jumps/day over 12 sessions; 100 jumps/day were performed for the remaining sessions Time: 20 min/session (5-min warm-up, 10-min jumping or stretching, 5-min cool down) Type: jumps off 61-cm box Control: nonimpact stretching |
DXA | Postintervention values | |||
| Significant | EX | CON | |||||||
| FN BMC | 2.00 ± 0.07*** | 1.89 ± 0.06 | |||||||
| FN BA | 3.13 ± 0.08*** | 2.96 ± 0.07 | |||||||
| LS BMC | 22.06 ± 0.57* | 21.64 ± 0.58 | |||||||
| LS aBMD | 0.571 ± 0.008** | 0.553 ± 0.008 | |||||||
| NS | EX group had significantly greater changes in FN and LS BMC than CON group (4.5 and 3.1 %, respectively). EX group also had significantly greater changes in FN BA (2.9 %) and LS aBMD (2.0 %) than CON group. | ||||||||
| FN aBMD, LS BA | |||||||||
| Nichols et al. 2001 [243] | 15-month resistance training intervention Randomized |
Sex: female Age: 14–17 years Race: not specified Location: Texas Woman’s University, TX |
16 | Frequency: 3×/week Intensity: progressive Time: not specified Type: 15 exercises all major muscle groups, combination free weights and machines Control: not specified |
DXA | Percent change, mean | |||
| Significant | EX | CON | |||||||
| FN aBMD | 3.67** | 1.35 | |||||||
|
NS
TB, LS, WA, TR aBMD TB, LS, FN, WA, TR BMC LS, FN BMAD |
FN aBMD increased significantly in EX group (40 %), but not in CON group. No significant changes seen at other sites | ||||||||
| Mackelvie et al. 2001 [26] | 9-month jumping intervention Randomized by school |
Sex: female Age: 9–12 years Race: multiethnic population of city (45 % white, 34 % Asian, and 21 % mixed ethnicities) reflected in cohort Location: Richmond, British Columbia |
177 | Frequency: 3×/week (2x PE, 1 classroom) Intensity: progressed over school year by increasing number of jumps per station (from 10 to 20) and height (from 10 to 50 cm) Time: 10–12 min (5 circuits, ~1.5–2 min each) Type: jumping exercises—jumping jacks, lunge jumps, hopping, jumping over various obstacles, drop jumps from platform Control: stretching |
DXA | Change, mean (95 % CI) | |||
| Significant | Prepubertal EX | Prepubertal CON | Early Pubertal EX | Early Pubertal CON | |||||
| LS BMC | 4.70 (4.38, 5.02)* | 4.18 (3.92, 4.44) | |||||||
| LS aBMD | 0.057 (0.050, 0.064)** | 0.044 (0.038, 0.049) | |||||||
| FN BMC | 0.31 (0.28, 0.34)* | 0.26 (0.24, 0.29) | |||||||
| FN aBMD | 0.043 (0.036, 0.050)* | 0.034 (0.028, 0.039) | |||||||
| FN vBMD | 0.010 (0.004, 0.015)* | 0.002(−0.003, 0.006) | |||||||
| NS | There were no significant differences between prepubertal EX and CON groups. Early pubertal girls in EX group gained 1.5 to 3.1 % more bone at FN and LS than CON girls; gain at other sites did not differ | ||||||||
| TB, PF, TR BMC | |||||||||
| TB, PF, TR aBMD | |||||||||
| Mackelvie et al. 2002 [339] | 7-month jumping intervention Randomized by school |
Sex: male Age: 8.8–11.7 years Race: multiethnic population of city (45 % white, 34 % Asian, and 21 % mixed ethnicities) reflected in cohort Location: Richmond, British Columbia |
121 | Frequency: 3×/week (2× PE, 1 classroom) Intensity: progressed over school year by increasing number of jumps per station (from 10 to 20) and height (from 10 to 50 cm) Time: 10–12 min (5 circuits, ~1.5–2 min each) Type: jumping exercises—jumping jacks, lunge jumps, hopping, jumping over various obstacles, drop jumps from platform Control: stretching |
DXA | Change, mean (95 % CI) | |||
| Significant | EX | CON | |||||||
| TB BMC | 105.8 (97.7, 113.9)** | 90.0 (81.9, 98.2) | |||||||
| PF aBMD | 0.025 (0.020, 0.030)* | 0.017 (0.013, 0.022) | |||||||
| NS | The EX group gained more TB BMC (1.6 %) and PF aBMD (1 %) than CON group did after adjusting for age, baseline weight, change in height, and loaded PA. | ||||||||
| LS, PF, FN, TR BMC | |||||||||
| LS, FN, TR aBMD | |||||||||
| Kontulainen et al. 2005 [125] | 20-month follow-up to 9-month step aerobics/jumping intervention Randomized by school |
Sex: female Age: 10–15 years Race: not specified Location: Tampere, Finland |
99 | Frequency: 2×/week Intensity: 100 both-leg jumps at floor level, increased to 200 box jumps (30 cm) using both legs and one leg Time: 50 min (10-min warm-up, 15-min nonimpact aerobic exercises, 20-min high-impact/jump training, 5-min cool down) Type: both-leg jumps at floor level, both-leg box jumps, one-leg box jumps Control: normal PA |
DXA | Percent difference, mean (95 % CI) | |||
| Significant | |||||||||
| LS BMC | 4.9 (0.9, 8.8)* | ||||||||
| NS | EX group had 4.9 % greater BMC accrual at the LS than the CON group during the 20-month follow-up. No other sites showed significant differences. | ||||||||
| FN, TR BMC | |||||||||
| Petit et al. 2002 [73] | 7-month jumping intervention Randomized by school |
Sex: female Baseline age: 9–12 years Race: multiethnic population of city (~34 % Hong Kong Chinese and 57 % white) reflected in cohort Location: Richmond, British Columbia |
177 | Frequency: 3×/week (2× PE, 1 classroom) Intensity: progressed over school year by increasing number of jumps per station (from 10 to 20) and height (from 10 to 50 cm) Time: 10–12 min (5 circuits, ~1.5–2 min each) Type: jumping exercises—jumping jacks, lung jumps, hopping, jumping over various obstacles, drop jumps from platform Control: stretching |
DXA | No significant differences seen between EX and CON groups | |||
| Significant | |||||||||
| None | |||||||||
| NS | |||||||||
| NN, IT, FS aBMD | |||||||||
| Laing et al. 2002 [340] | 36-month gymnastics participation Recruited due to enrollment in gymnastics |
Sex: female Age: 8–13 years at baseline Race: all Caucasian (except one Hispanic control) Location: Athens, Georgia |
17 | Frequency: not specified Intensity: not specified Time: 9–24 h/week Type: regionally competitive gymnastics: vault, uneven bars, balance beam, floor routine Control: no gymnastics |
DXA | At year 3, significant differences between EX and CON were noted at TB, LS, and PF for aBMD. Significant differences were noted for TB and LS for BMC. Both the EX group and CON groups had significant increases in BA at the TB, LS, FN, and PF. | |||
| Significant | |||||||||
| TB aBMD | |||||||||
| LS aBMD | |||||||||
| PF aBMD | |||||||||
| TB BMC | |||||||||
| LS BMC | |||||||||
| TB BA | |||||||||
| LS BA | |||||||||
| FN BA | |||||||||
| PF BA | |||||||||
| NS | |||||||||
| FN, TR, Rad aBMD | |||||||||
| PF, FN, TR, Rad BMC | |||||||||
| FN, Rad BA | |||||||||
| MacKelvie et al. 2003 [341] | 20-month jumping intervention Randomized by school |
Sex: female Age: 8.8–11.7 years at baseline Race: multiethnic population of city (57 % white, 34 % Hong Kong Chinese, 5 % East Indian, 4 % other ethnic origin or mixed ethnicity) reflected in the cohort Location: Richmond, British Columbia |
75 | Frequency: 3×/week (2× PE, 1 classroom) Intensity: year 1: progressed over school year by increasing number of jumps per station (from 10 to 20) and height (from 10 to 50 cm); year 2: higher proportion of high-impact jumps. Height increased every 8–10 weeks Time: 10–12 min (5 circuits, ~1.5–2 min each) Type: year 1: jumping exercises such as jumping jacks, lung jumps, hopping, jumping over various obstacles, and drop jumps from platform; year 2: plyometric jumps, alternating-foot jumps, and 2-ft obstacle jumps Control: stretching |
DXA | Change, mean (95 % CI) | |||
| Significant | EX | CON | |||||||
| LS BMC | 10.9 (9.9, 11.9)* | 9.3 (8.4, 10.1) | |||||||
| FN BMC | 0.66 (0.58, 0.73)* | 0.55 (0.48, 0.61) | |||||||
| NS | There were substantially greater gains in LS (41.7 vs 38.0 %) and FN (24.8 vs 20.2 %) BMC in EX group than in CON group. No significant differences between groups at other sites. | ||||||||
| TB, PF, TR BMC | |||||||||
| LS, PF, FN, TR BA | |||||||||
| Van Langendonck et al. 2003 [342] | 9-month impact intervention on twins Twins randomized to exercise or control, keeping birth order equal between groups |
Sex: female (21 twin pairs) Age: 8.7 years (SD = 0.7) Race: not specified Location: Belgium |
42 | Frequency: 3×/week Intensity: after the first trimester, participants went barefoot or wore very thin gym shoes to enhance mechanical loading Time: 10 min/session Type: 3 impact exercises- rope skipping 50 times; hopping 20 times as far as possible with the left and right leg; jumping from a wooden box of 40 cm high landing on both feet 30 times. 2 nonimpact exercises- moving 3.5 m sideways in a push-up position six times and six times moving 3.5 m backward on hands and feet like a crab Control: asked not to perform the exercises at school or home |
DXA | None of the bone indices differed significantly between the EX and CON groups after the 9-month intervention. | |||
| Significant | |||||||||
| None | |||||||||
| NS | |||||||||
| LS, FN, PF, RA, TB BMC | |||||||||
| LS, FN, PF, RA, TB aBMD | |||||||||
| LS, FN, PF, RA, TB BA | |||||||||
| Iuliano-Burns et al. 2003 [155] | 8.5-month randomized intervention trials with two factors (exercise and calcium) that each had two levels | Sex: female Age: 7–11 years Race: 15 % Asian descent Location: Melbourne, Australia |
66 | Frequency: 3×/week (PE) Intensity: moderate (2–4× body wt GRF) Time: 20 min/session Type: hopping-, jumping-, and skipping-based activities Control: stretching and low-impact dance routines (1× body wt) |
DXA | Change, mean ± SEM | |||
| EX | CON | ||||||||
| Significant | Calcium | Placebo | Calcium | Placebo | |||||
| Leg BMC | 58.9 ± 7.1b | 50.1 ± 3.3b | |||||||
| Femur BMC | 29.9 ± 4.1b | 24.1 ± 2.0b | |||||||
| T-F BMC | 21.1 ± 1.8d,f | 17.1 ± 1.3d | 18.3 ± 2.2f | ||||||
| Arm BMC | 15.5 ± 1.9a,c | 10.3 ± 1.3c,d | 13.1 ± 0.91d | 10.8 ± 1.3a | |||||
| Hum BMC | 7.3 ± 0.9c | 5.2 ± 0.5c | |||||||
| U-R BMC | 4.4 ± 0.5a,c | 3.1 ± 0.4c,d | 4.7 ± 0.4d,e | 3.3 ± 0.4a,c | |||||
| NS | Like letters denote significant difference between groups (P < 0.05). An exercise-calcium interaction was detected at the femur (7.1 %). By contrast, there was no exercise–calcium interaction detected at the T-F; however, there was a main effect of exercise: BMC increased 2–4 % more in the calcium-supplemented groups than in the nonsupplemented groups at the humerus (12.0 vs 9.8 %) and R-U (12.6 vs 8.6 %). |
||||||||
| TB, LS BMC | |||||||||
| Specker and Binkley 2003 [81] | 1-year randomized, placebo-controlled, partially blinded trial of PA and calcium supplementation | Sex: male and female Age: 3–5 years Race: 94 % white, 6 % other Location: South Dakota |
178 | Frequency: 5×/wk Intensity: not specified Time: 30 min/day (5-min warm-up, 20-min activity, 5-min cool down) Type: hopping, jumping, skipping activities (17 different weekly programs) Control: 30 min/day of activities to keep them sitting quietly |
DXA | Change, mean ± SEM | |||
| Significant | EX | CON | |||||||
| Calcium | Placebo | Calcium | Placebo | ||||||
| Leg BMC | 40.9 ± 1.3 | 38.2 ± 1.2 | 37.3 ± 1.4 | 38.5 ± 1.3 | |||||
| NS | There was a significant interaction (P < 0.05) between the activity and supplement groups in leg BMC gain; the difference in BMC gain between EX and CON groups was more pronounced in children receiving calcium vs placebo. Among children receiving the placebo, leg BMC gain was similar in the EX and CON groups. However, among children receiving calcium, those in EX group has 9.7 % greater increase in leg BMC than those in CON group. | ||||||||
| TB, arm BMC | |||||||||
| TB, arm, leg BA | |||||||||
| Stear et al. 2003 [156] | 15.5-month randomized, double-blind trial of PA and calcium supplementation | Sex: female Age: 17.3 ± 0.4 years Race: not listed Location: UK |
131 | Frequency: 3×/wk Intensity: moderate-to-vigorous intensity, moderate-to-high impact Time: 45 min/session (7- to 10-min warm-up, 30-min workout, 5- to 8-min warm-down and stretch) Type: exercise-to-music aerobics class Control: not invited to exercise sessions |
DXA | No significant differences seen between EX and CON groups | |||
| Significant | |||||||||
| None | |||||||||
| NS | |||||||||
| TB, LS, Rad, R-Ul, R-Dis BMC | |||||||||
| TB, LS, Rad, R-Ul, R-Dis SA-BMC | |||||||||
| MacKelvie et al. 2004 [295] | 20-month jumping intervention Randomized by school |
Sex: male Age: 8.8–12.1 years Race: multiethnic population of city (~34 % Hong Kong Chinese and 57 % North American/Western European Caucasian, 5 % Southeast Asian, and 4 % other or mixed ethnicity) reflected in cohort Location: Richmond, British Columbia |
64 | Frequency: 3×/wk (2× PE, 1 classroom) Intensity: progressed over 2 years by increasing number of jumps per station (from 10 to 20) and height (from 10 to 50 cm Time: 10–12 min (5 circuits, ~1.5–2 min each) Type: jumping exercises such as jumping jacks, lung jumps, hopping, jumping over various obstacles, drop jumps from platform Control: stretching |
DXA | Change, mean (95 % CI) | |||
| Significant | EX | CON | |||||||
| FN BMC | 0.50 (0.45, 0.55)** | 0.39 (0.34, 0.43) | |||||||
| TB, LS, PF, FN, TR BA | |||||||||
| NS | FN BMC changes were significantly greater in EX boys (4.3 %); changes in bone area and BMC for other regions were not significantly different between groups. | ||||||||
| TB, LS, PF, TR BMC | |||||||||
| McKay et al. 2005 [297] | 8-month jumping intervention Control group used from previous study |
Sex: male and female Age: 8.9 to 11.0 years Race: 38 % Caucasian, 48 % Asian, 15 % other (including mixed ethnic, black, and South Asian) Location: Richmond, British Columbia |
124 | Frequency: 3×/days Intensity: 5× body wt, maximum rate of force was >400 body wt/s (independent sample of 70 boys and girls) Time: 3 min/day Type: 10 counter movement jumps (two-foot take off, clutch knees, two-foot landing) Control: not specified, from previous study |
DXA | Change, mean (95 % CI) | |||
| Significant | EX | CON | |||||||
| TB BMC | 92.7 (83, 102)* | 106 (98, 114) | |||||||
| TB BA | 76.7 (66.9, 86.5)* | 95.6 (87.6, 103.6) | |||||||
| PF BMC | 2.3 (2.1, 2.6)* | 1.9 (1.7, 2.1) | |||||||
| IT BMC | 1.5 (1.3, 1.7)* | 1.2 (1.0, 1.3) | |||||||
| NS | CON group had greater increase in adjusted TB BMC (1.4 %). EX group gained significantly more BMC at the PF (2 %) and at the IT region (27 %). | ||||||||
| LS, PF, IT, GT, FN BA | |||||||||
| LS, GT, FN BMC | |||||||||
| Laing et al. 2005 [343] | 24-month quasi-experimental prospective gymnastics study Recruited due to enrollment in gymnastics |
Sex: female Age: 4–8 years Race: 64 % white, 27 % black, 3 % Asian, 2 % Hispanic, 1 % Indian, and 3 % other Location: Athens, Georgia |
143 | Frequency: 1×/week Intensity: not specified Time: 1 h/session Type: recreational gymnastics class Control: participating in nongymnastic activities or no activities |
DXA | Postintervention values, mean ± SD | |||
| Significant | EX | CON | |||||||
| TB BA | 1392 ± 247* | 1545 ± 314 | |||||||
| TB BMC | 997 ± 255* | 1151 ± 323 | |||||||
| TB aBMD | 0.708 ± 0.06* | 0.736 ± 0.07 | |||||||
| LS BA | 34.1 ± 50.5* | 36.6 ± 6.17 | |||||||
| LS BMC | 20.5 ± 5.47* | 22.6 ± 6.24 | |||||||
| PF BA | 18.8 ± 4.04* | 20.4 ± 4.53 | |||||||
| PF BMC | 12.5 ± 4.07* | 14.1 ± 4.56 | |||||||
| FA BA | 7.39 ± 1.58* | 8.05 ± 1.80 | |||||||
| FA BMC | 2.85 ± 0.85* | 3.19 ± 0.97 | |||||||
| NS | Values suggest that EX remained lower in bone mineral measures after 2 years; however, after adjusting for initial differences, it was determined that EX had greater BA, BMC, and aBMD responses compared with CON at some skeletal sites. | ||||||||
| LS, PF, FA aBMD | |||||||||
| Yu et al. 2005 [242] | 36-week strength training intervention Randomized |
Sex: male and female Age: 9–11 years Race: not specified Location: Hong Kong |
63 | Frequency: phase 1: 3×/week; phase 2: 1×/week Intensity: phase 1: 75 % 10 RM, increased to 100 % over time. 1 set, 20 reps; phase 2: weight adjusted according to ability. 2–3 sets of 10–20 reps Time: phase 1: 75 min/session (10-min warm-up, 30-min strength training, 10-min agility training, 5-min cool down); phase 2: 60 min/session (5-min warm-up, 10-min stretching, 40-min strength training, 5-min cool down) Type: phase 1: circuits (9 stations for strength training, 1 station for agility, 1 aerobic station); phase 2: similar to phase 1 Control: hypocaloric diet, no strength training |
DXA | No significant differences in BMC were observed between the EX and CON groups at 36 weeks. | |||
| Significant | |||||||||
| None | |||||||||
| NS: | |||||||||
| TB, TH, LS BMC | |||||||||
| Valdimarsson et al. 2006 [344] | 1-year expanded PE intervention Randomized by school |
Sex: female Age: 6.5–8.9 years Race: Caucasian Location: Malmö, Sweden |
103 | Frequency: 5 days/week Intensity: varied Time: 40 min/day Type: normal PE curriculum (running, jumping, climbing ropes, variety of ball games). Activities varied to reduce boredom. No specific osteogenic training program was added. Control: normal PE curriculum, duration within normal limits (1–2 sessions/week, in total 60 min/week) |
DXA | Change, mean (SD) | |||
| Significant | EX | CON | |||||||
| L2-L4 BMC | 2.4 (1.1)** | 1.8 (0.7) | |||||||
| L3 BMC | 0.94 (0.63)*** | 0.53 (0.30) | |||||||
| L2-L4 aBMD | 0.044 (0.03)*** | 0.026 (0.01) | |||||||
| L3 aBMD | 0.047 (0.03)*** | 0.025 (0.02) | |||||||
| L3 width | 0.17 (0.12)*** | 0.09 (0.06) | |||||||
| NS | The annual gain in BMC was 4.7 % higher in the LS and 9.5 % higher in L3 in the EX group than in the CON group. The annual gain in aBMD was 2.8 % higher in the LS and 3.1 % higher in L3 in the EX group than in the CON group. The annual gain in L3 width was 2.9 % higher in the EX group than in the CON group. | ||||||||
| TB, FN, leg BMC | |||||||||
| TB, FN, leg aBMD | |||||||||
| L3, FN vBMD | |||||||||
| FN width | |||||||||
| Linden et al. 2006 [345] | 2-year expanded PE intervention Randomized by school |
Sex: female Age: 6.5–8.9 years Race: white Location: Malmö, Sweden |
99 | Frequency: 5 days/week Intensity: varied Time: 40 min/day Type: normal PE curriculum (running, jumping, climbing ropes, variety of ball games). Activities varied to reduce boredom. No specific osteogenic training program was added. Control: normal PE curriculum, duration within normal limits (1–2 sessions/week, in total 60 min/week) |
DXA | Change, mean | |||
| Significant | EX | CONZ | |||||||
| L2-L4 BMC | 2.2* | 1.8 | |||||||
| L3 BMC | 0.8*** | 0.6 | |||||||
| L2-L4 aBMD | 0.034* | 0.026 | |||||||
| FN vBMD | −0.004** | 0.006 | |||||||
| L3 width | 0.136** | 0.092 | |||||||
| NS | The annual gain in BMC was greater in the EX group than in the CON group: L2-L4 3.8 %, L3 7.2 %. The EX group had greater annual gain in aBMD at L2-L4 (1.2 %). There was also a greater mean annual gain in bone size in the L3 vertebra (1.8 %). | ||||||||
| TB, FN, leg BMC | |||||||||
| TB, L3, FN, leg aBMD | |||||||||
| L3 vBMD | |||||||||
| FN width | |||||||||
| Bass et al. 2007 [157] | 8.5-month randomized intervention trials with two factors (exercise and calcium) that each had two levels | Sex: male Age: 7–11 years Race: 22 % Asian descent Location: Melbourne, Australia |
88 | Frequency: 3×/week (PE) Intensity: moderate (2–8× body wt GRF) Time: 20 min/session Type: hopping-, jumping-, and skipping-based activities Control: stretching and low-impact dance routines (1× body wt) |
DXA | Change, mean (SD) | |||
| EX | CON | ||||||||
| Significant | Calcium | Placebo | Calcium | Placebo | |||||
| Leg BMC | 64.4 (4.2)abc | 54.0 (4.7)a | 47.7 (4.2)b | 43.3 (3.2)c | |||||
| Femur BMC | 30.1 (1.8)abc | 24.9 (1.9)a | 24.8 (1.4)b | 23.1 (1.3)c | |||||
| T-F | 23.4 (2.1)a | 16.8 (1.5)a | |||||||
| NS | Like letters denote significant difference between groups (P < 0.05). The increase in femur BMC in the EX + calcium group was ~2 % greater than the increase in the EX + placebo, no-EX + calcium, or no-EX + placebo groups. At the T-F, the increase in BMC in the EX + calcium group was ~3 % greater than in the no-EX + placebo group |
||||||||
| TB, LS, arm, Hum, U-R BMC | |||||||||
| Linden et al. 2007 [298] | 1-year expanded PE intervention Randomized by school |
Sex: male Age: 6.7–9.0 years Race: Caucasian (except 1 boy adopted from Colombia) Location: Malmö, Sweden |
138 | Frequency: 5 days/week Intensity: varied Time: 40 min/day Type: normal PE curriculum (running, jumping, climbing ropes, variety of ball games). Activities varied to reduce boredom. No specific osteogenic training program was added. Control: normal PE curriculum, duration within normal limits (1–2 sessions/week, in total 60 min/week) |
DXA | Change, mean (SD) | |||
| Significant | EX | CON | |||||||
| L3 BMC | 0.87 (0.55)*** | 0.58 (0.26) | |||||||
| L3 aBMD | 0.041 (0.04)** | 0.027 (0.02) | |||||||
| L3 width | 0.14 (0.14)*** | 0.07 (0.07) | |||||||
| NS | The mean annual gain in L3 BMC was 5.9 % higher, L3 aBMD a mean 2.1 % higher, and L3 width a mean 2.3 % higher in the EX group than in the CON group. | ||||||||
| TB, FN BMC | |||||||||
| TB FN aBMD | |||||||||
| L3, FN vBMD | |||||||||
| FN width | |||||||||
| Barbeau et al. 2007 [346] | 10-month MVPA intervention Randomized |
Sex: female Age: 8–12 years Race: black Location: Georgia |
201 | Frequency: every day school in session during school year; during summer Intensity: HR > 150 bpm during MVPA activity (HR monitor) Time: 110-min after-school program (80 min of PA) Type: 25-min skills development (dribbling basketball), 35-min MVPA, 20-min toning and stretching Control: no intervention |
DXA | Difference, mean (95 % CI) | |||
| Significant | |||||||||
| BMC | 0.044 (0.024, 0.064)*** | ||||||||
| aBMD | 0.020 (0.012, 0.027)*** | ||||||||
| Compared to the CON group, the EX group had a greater increase in BMC and aBMD. | |||||||||
| Schneider et al. 2007 [347] | 1-year MVPA intervention Randomized by school |
Sex: female Age: 15.04 years (SD = 0.79) Race: 57 % non-Hispanic white, 20 % Hispanic, 17 % Asian, 6 % other Location: 2 public high schools, location not specified |
122 | Frequency: 5 days/week Intensity: varied Time: 60 min/day Type: supervised activities included variety of aerobic (3×/week, including aerobic dance, kickboxing, and brisk walking) and strength-building (1×/week, including weightlifting and yoga) activities Control: no intervention |
DXA | Percent change, mean | |||
| Significant | EX | CON | |||||||
| TS BMC | 6.3** | 1.4 | |||||||
| NS | The effect of EX on BMC for the TS was significant and increased 6.3 % as opposed to 1.4 % in the CON group. None of the other sites for BMC or any aBMD measures were significant. | ||||||||
| TB, LS, FN, TH, TR BMC | |||||||||
| TB, LS, FN, TH, TS, TR aBMD | |||||||||
| Gunter et al. 2008 [24] | 7-month jumping intervention Randomized by school |
Sex: male and female Age: 7–10 years Race: not specified Location: Corvallis, OR |
56 | Frequency: 3×/week, during PE Intensity: Progressed to reach 90–100 jumps per session Time: 30 min/session (warm-up, fitness development (jumping), lesson focus, closing activity) Type: jumping Control: similar class structure, no jumping during fitness development |
DXA | Postintervention values, mean (SD) | |||
| Male | Female | ||||||||
| Significant | EX | CON | EX | CON | |||||
| TH BMC | 28.5 (9.2)a | 25.7 (7.56)a | 24.6 (6.2)a | 24.3 (6.1)a | |||||
| FN BMC | 3.8 (0.96)b | 3.6 (0.77)b | 3.3 (0.74)b | 3.3 (0.71)b | |||||
| Other variables |
aMales greater than females (P < 0.05); bmales greater than females (P < 0.01) Three years after the intervention had concluded, the EX group had 2.3, 3.2, 4.4, and 2.9 % greater BMC than controls at the LS, TH, FN, and TB, respectively. |
||||||||
| TB, LS, TR BMC | |||||||||
| Gunter et al. 2008 [24] | 7-month jumping intervention Randomized by school |
Sex: male and female Age: 7–10 years Race: 47 white, 2 Asian Location: Corvallis, OR |
49 | Frequency: 3×/week, opposite PE Intensity: progressed to reach 100 jumps/session by the 5th wk of the program Time: 20 min/session (5-min warm-up, 10-min jumping or stretching, 5-min cool down) Type: jumps off 24 in box Control: nonimpact stretching |
DXA | Postintervention values, mean (SD) | |||
| Significant | Males | Females | |||||||
| EX | CON | EX | CON | ||||||
| TH BMC | 39.93 (9.56)a | 39.31 (9.93) | 31.26 (5.40)a | 30.15 (3.38) | |||||
| FN BMC | 4.92 (0.88)a | 4.73 (0.93) | 4.18 (0.53)a | 4.21 (0.69) | |||||
| TR BMC | 11.11 (2.62)a | 10.91 (2.54) | 8.31 (1.42)a | 7.49 (1.05) | |||||
|
aMales significantly greater than females for all values (P < 0.01). Participants in the EX group had 1.4 % more TH BMC than those in the CON group after 8 years |
|||||||||
| Weeks et al. 2008 [296] | 8-month jumping intervention Randomized |
Sex: male and female Age: boys 13.8 years (0.4); girls 13.7 years (0.5) Race: not specified Location: Gold Coast, Australia |
81 | Frequency: 2×/week Intensity: 1–3 Hz at a height of 0.2–0.4 m Time: 10 min (~300 jumps) Type: varied, but included jumps, hops, tuck jumps, jump squats, stride jumps, star jumps, lunges, side lunges, and skipping. Occasionally supplemented with upper body strengthening activities, including push-ups and exercises with resistive latex bands Control: usual PE warm-up and stretching |
DXA | Girls in the EX group improved FN BMC (13.9 %) more than those in the CON group. Between-group comparisons of change showed EX group effects only for TB BMC (10.6 %) for boys. Boys in the EX group gained more TR BMC, LS BMC, and TB BMC than girls in the EX group. | |||
| Significant | |||||||||
| FN BMC | |||||||||
| TR BMC | |||||||||
| TB BMC | |||||||||
| NS | |||||||||
| FN, LS BA | |||||||||
| LS BMC | |||||||||
| Macdonald et al. 2008 [299] | 11-month PA and jumping intervention Randomized by school |
Sex: male and female Age: 9–11 years Race: 53 % Asian (both parents or all four grandparents born in Hong Kong or China, India, Philippines, Vietnam, Korea, or Taiwan. 35 % Caucasian (parents born in North America or Europe). 12 % children mixed ethnicity or other ethnic origins Location: Vancouver and Richmond, British Columbia |
410 | Frequency: Classroom Action: 5 days/week; Bounce at the Bell: 5 days/week PA + 3×/day, 4 days/week jumping Intensity: not specified Time: Classroom Action: 15 min PA; Bounce at the Bell: 15 min PA + short bouts high-impact jumping Type: Classroom Action: skipping, dancing, playground circuits, simple resistance exercises with bands; Bounce at the Bell: Classroom Action + short bouts high-impact jumping. Phase I included 5 two-foot landing jumps (or 10 one-foot landing jumps) at each session. Phase II jumps were increased each month of the school year until a maximum of 36 jumps/day was reached. Control: usual PE |
DXA | Percent difference, mean (95 % CI) | |||
| Significant | Males | Females | |||||||
| FN BMC | 0.96 (−0.003, 1.93)* | 0.09 (0.005, 0.172)** | |||||||
| LS BMC | 30.8 (7.2, 54.4)** | ||||||||
| TB BMC | |||||||||
| NS | Boys in EX group had greater gains in BMC at the LS (2.7 %) and TB (1.7 %) than the controls did. Girls in the EX group had greater gains in FN BMC (3.5 %) than in controls. | ||||||||
| PF BMC | |||||||||
| Alwis et al. 2008 [300] | 1-year expanded PE intervention Randomized by school |
Sex: female Age: 6.5–8.9 years Race: Caucasian Location: Malmö, Sweden |
103 | Frequency: 5 days/week Intensity: varied Time: 40 min/day Type: normal PE curriculum (running, jumping, climbing ropes, variety of ball games). Activities varied to reduce boredom. No specific osteogenic training program was added. Control: normal PE curriculum, duration within normal limits (1–2 sessions/week, in total 60 min/week) |
DXA | No between-group differences were found during the 12-month study period for changes in FN variables. | |||
| Significant | |||||||||
| None | |||||||||
| NS | |||||||||
| FN BMC, aBMD, vBMD | |||||||||
| Alwis et al. 2008 [300] | 2-year expanded PE intervention Randomized by school |
Sex: male Age: 6.7–9.0 years Race: Caucasian Location: Malmö, Sweden |
137 | Frequency: 5 days/week Intensity: varied Time: 40 min/day Type: normal PE curriculum (running, jumping, climbing ropes, variety of ball games). Activities varied to reduce boredom. No specific osteogenic training program was added. Control: normal PE curriculum, duration within normal limits (1–2 sessions/week, in total 60 min/week) |
DXA | Change, mean (SD) | |||
| Significant | EX | CON | |||||||
| L3 BMC | 0.72 (0.30)** | 0.58 (0.26) | |||||||
| L3 width | 0.11 (0.07)** | 0.07 (0.07) | |||||||
| NS | The mean annual gain in L3 BMC (3 %) and L3 width (1.3 %) were greater in the EX group than in the CON group . | ||||||||
| TB, FN BMC | |||||||||
| FN width | |||||||||
| Alwis et al. 2008 [300] | 2-year expanded PE intervention Randomized by school |
Sex: female Age: 6.8–8.9 years Race: Caucasian Location: Malmö, Sweden |
83 | Frequency: 5 days/week Intensity: varied Time: 40 min/day Type: normal PE curriculum (running, jumping, climbing ropes, variety of ball games). Activities varied to reduce boredom. No specific osteogenic training program was added. Control: normal PE curriculum, duration within normal limits (1–2 sessions/week, in total 60 min/week) |
DXA | No between-group differences were observed for annual changes in the FN variables. | |||
| Significant | |||||||||
| None | |||||||||
| NS | |||||||||
| FN BMC, aBMD | |||||||||
| Meyer et al. 2011 [348] | 9-month additional PE session intervention Randomized by class |
Sex: male and female Age: prepubertal = 6–7 years; early pubertal = 11–12 years Race: not specified Location: Aargau and Baselland, Switzerland |
291 | Frequency: 2×/week (PE); 3×/day (activity breaks); 1×/day (home activity) Intensity: not specified Time: 45 min/session (PE); 2–5 min/session (activity breaks); 10 min/day (home activity) Type: Two PE lessons (plus 3 regular PE classes) taught mostly outdoors by PE teachers. All five sessions included jumping activities like hopping, jumping up and down stairs, rope skipping, etc. During academic lessons, 3–5 activity breaks comprised motor skill tasks such as jumping around on one leg, balancing on one leg, power games, or coordinative tasks were introduced every day. Daily home activity included aerobic, strength, or motor skill tasks like tooth brushing while standing on one leg, jumping up and down the stairs, and rope jumping. Control: participated in regular PE classes 3×/week |
DXA | Difference, Z-score (95 % CI) | |||
| Significant | EX | ||||||||
| TB BMC | 0.138 (0.06, 0.216)*** | ||||||||
| FN BMC | 0.136 (0.008, 0.264)* | ||||||||
| LS BMC | 0.118 (0.028, 0.2017)** | ||||||||
| TB aBMD | 0.212 (0.088, 0.337)*** | ||||||||
| LS aBMD | 0.184 (0.083, 0.285)*** | ||||||||
| NS | Compared to CON group, children in EX group showed statistically significant increases in BMC of TB (5.5 %), FN (5.4 %), and LS (4.7 %) and aBMD of TB (8.4 %) and LS (7.3 %) | ||||||||
| FN aBMD | |||||||||
| Silva et al. 2011 [349] | Sports study Recruited based on sport participation |
Sex: male Age: 10–18 years Race: not specified Location: Brazil |
46 | Inclusion criteria: 3 year experience in sport, training at least 10 h/week in previous 6 months, only PA associated with sport, high competitive level Control: normal PE |
DXA | aBMD, mean (SD) | |||
| Significant | Tennis | Soccer | Swimming | Controls | |||||
| PF aBMD | 1.02 (0.18)* | 1.08 (0.16)* | |||||||
| NS | Results showed higher mean values in the PF region of tennis and soccer players than of swimmers and controls. In relation to the impact of sporting activities based on bone age determination, a significant difference in aBMD was observed at all evaluated sites at the end of puberty (16–18 years) compared with 10–12 years, with increases of 78 % at the LS, 37 % at the PF, and 38 % TB. | ||||||||
| LS, TB aBMD | |||||||||
| Lofgren et al. 2012 [28] | 4-year expanded PE intervention Randomized by school |
Sex: male and female Age: 6.5–8.7 years Race: Caucasian Location: Malmö, Sweden |
221 | Frequency: 5 days/week Intensity: varied Time: 40 min/day Type: normal PE curriculum (running, jumping, climbing ropes, variety of ball games). Activities varied to reduce boredom. No specific osteogenic training program was added Control: normal PE curriculum, duration within normal limits (1–2 sessions/week, in total 60 min/week) |
DXA | Change, mean (95 % CI) | |||
| Significant | Male | Female | |||||||
| EX | CON | EX | CON | ||||||
| TB BMC | 179.6 (160.5, 198.7)* | 166.4 (149.4, 183.4) | |||||||
| LS BMC | 7.0 (6.5, 7.6)* | 6.2 (5.8, 6.6) | 9.1 (7.9, 10.3)** | 7.1 (6.1, 8.0) | |||||
| FN BMC | 0.29 (0.26, 0.31) | 0.27 (0.24, 0.31) | 0.39 (0.33, 0.45)** | 0.28 (0.23, 0.33) | |||||
| TR BMC | 0.92 (0.82, 1.02)** | 0.72 (0.62, 0.83) | |||||||
| L3 width | 0.13 (0.11, 0.14)** | 0.11 (0.09, 0.12) | |||||||
| FN width | 0.12 (0.11, 0.13)* | 0.11 (0.09, 0.12) | 0.15 (0.12, 0.17)** | 0.10 (0.08, 0.13) | |||||
| The mean annual gain in LS BMC was 7.0 % higher in girls and 3.3 % higher in boys, and in FN width 1.7 % higher in girls and 0.6 % higher in boys in the EX group than in the CON group. | |||||||||
| Detter et al. 2014 [303] | 6-year expanded PE intervention Randomized by school |
Sex: male and female Age: 6–8 years Race: Caucasian Location: Malmö, Sweden |
295 | Frequency: 5 days/week Intensity: varied Time: 40 min/day Type: normal PE curriculum (running, jumping, climbing ropes, variety of ball games). Activities varied to reduce boredom. No specific osteogenic training program was added, Control: normal PE curriculum, duration within normal limits (1–2 sessions/week, in total 60 min/week) |
DXA | Difference, mean (95 % CI) | |||
| Significant | Male | Female | |||||||
| LS aBMD | 0.006 (0.002, 0.01)** | 0.01 (0.003, 0.02)* | |||||||
| FN BMC | 0.07 (0.01, 0.12)* | ||||||||
| NS | Girls in the EX group, compared with girls in the CON group, had 0.009 g/cm2 larger gain annually in LS aBMD and 0.07 g larger gain in FN BMC. Boys in the EX group has 0.006 g/cm2 larger gain annually in LS aBMD than CON boys, | ||||||||
| TB, FN aBMD | |||||||||
| TB, LS BMC | |||||||||
| FN, LS BA | |||||||||
| Observational studies | |||||||||
| Reference | Study description | Population description | N | Exposure | End points | Results | |||
| Lehtonen-Veromaa et al. 2000 [350] | 1-year prospective case comparison | Sex: 155 females Baseline age: 9–15 years Race: white Location: Turku, Finland |
51 gymnasts, 50 runners, 54 controls | Method: self-report questionnaire (2×) Variable: leisure-time PA met (h/week) |
Follow-up TH (and subregions), LS aBMD, and BA | The 1-year increase in adjusted aBMD at the FN of the gymnasts was 115 % larger than that of the controls and 125 % larger than that of the runners. The 1-year increase in adjusted aBMD at the TR of the gymnasts was 49 % larger than that of controls. | |||
| Molgaard et al. 2001 [245] | 1-year prospective follow-up | Sex: 140 males, 192 females Baseline age: 5–19 years Race: white Location: Copenhagen, Denmark |
332 | Method: 24-h recall questionnaire (3×) (I, supine; II, sitting; III, walking; IV, breathless PA) Variable: breathless PA (h/day) |
Follow-up TB BMC, BA | Breathless PA not associated with BMC or BA | |||
| Gustavsson et al. 2003 [351] | 2.5-year and 6-year prospective case comparison | Sex: 68 males Baseline age: 16 years Race: white Location: Umea, Sweden |
22 hockey, 21 retired hockey, 25 controls | Method: reported participation in ice hockey Variable: group membership: hockey, retired hockey, no hockey |
Follow-up at 30 months (~2.5 years) and follow-up at 70 months (~6 years) TB, FN, LS aBMD | At 30 months, hockey players had greater gains in aBMD FN than controls (0.07 vs 0.03 g/cm2). Hockey players had significantly higher aBMD at FN and TB than controls. At 70 months, hockey players had greater aBMD at the FN, TB, and LS compared to nonplayers. Retired hockey players still had 4 % higher aBMD of FN than nonplayers after 70 months | |||
| Nurmi-Lawton et al. 2004 [352] | 3-year prospective case comparison | Sex: 97 females Baseline age: 7.9–17.2 years Race: primarily white Location: England |
45 gymnasts, 52 controls | Method: reported participation in gymnastics Variable: group membership gymnastics, no gymnastics |
Multilevel model comparing differences in TB, LS, arm, and leg BMC, as well as aBMD each year | Gymnasts had 24–51 % greater BMC and 13–28 % greater aBMD than controls. | |||
| Laing et al. 2005 [343] | 2-year quasi-experimental | Sex: 143 females Baseline age: 4–8 years Race: 64 % white, 27 % Black, 3 % Asian, 2 % Hispanic, 4 % other Location: Athens, Georgia |
65 gymnasts, 78 controls | Method: reported participation in gymnastics Variables: group membership gymnastics, no gymnastics, and h gymnastics participation per week |
Follow-up TB, LS, PF, forearm BMC, aBMD, and BA | LS aBMD and forearm BA increased at a greater rate in gymnasts than in controls. Forearm BA increased at a greater rate in high-hours-per-week gymnastics over low-hours-per-week gymnastics | |||
| Janz et al. 2006 [353] | 3-year prospective follow-up | Sex: 171 males, 199 females Baseline age: 5 years Race: primarily white Location: Iowa, USA |
370 | Method: ActiGraph accelerometer Variables: total PA ct/min, active min/day (≥3000 ct/min) |
Follow-up TB, TH, TR, and LS BMC | PA was significantly associated with TB, TH, TR, and LS BMC in males. In females, PA was associated with TB and TR BMC. PA explained 1–2 % of variance in BMC. | |||
| Nordstrom et al. 2006 [354] | ~8-year prospective case comparison | Sex: 90 males Baseline age: 15–19 years Race: Caucasian Location: Umea, Sweden |
63 athletes, 27 controls |
Method: reported participation in ice hockey or badminton Variables: weight-bearing PA (h/week) |
Follow-up and sustained effects TB, FN, TH, and Hum BMD; FN BA, TH BA | Active athletes had significantly higher BMD at all measured sites vs controls (P < 0.05). Former athletes still had higher BMD of the FN, TH, and Hum than did controls (P < 0.05). | |||
| Baxter-Jones et al. 2008 [22] | ~15-year prospective follow-up | Sex: 72 males, 82 females Baseline age: 8–15 years Race: not specified Location: Saskatoon, Saskatchewan, Canada |
154 | Method: self-report questionnaire (3×/year for 3 years, 2×/year after) Variable: general level of PA for year (1–5) |
Follow-up (age 23–30 years) TB, LS, TH, and FN BMC | In young adulthood, males who were physically active as adolescents had 8–9 % greater BMC at TB, TH, and FN compared to inactive adolescent males. In young adulthood, females who were active adolescents had 9 % greater TH and 10 % greater FN compared to inactive adolescent females | |||
| Cheng et al. 2009 [54] | 7-year prospective follow-up | Sex: 396 females Baseline age: 10–13 years Race: not specified Location: Jyvaskyla, Finland |
396 | Method: self-report leisure-time PA questionnaire with parent help Variables: leisure-time PA score |
Follow-up TB BMC | PA did not contribute to variability in BMC. | |||
| Janz et al. 2010 [355] | 3-year and 6-year prospective follow-up | Sex: 148 males, 185 females Baseline age: 5 years Race: primarily white Location: Iowa, USA |
333 | Method: ActiGraph accelerometer Variables: MVPA min/day (>3000 ct/min) |
Follow-up at age 8 and age 11 TB, TH, and LS BMC | After adjustment for concurrent (age 8 or 11) age, height, weight, somatic maturity, and MVPA, age 5 MVPA was significant predictor of ages 8 and 11 BMC in boys and girls at TB, TH, and LS. | |||
| Tervo et al. 2010 [356] | ~12-year case comparison | Sex: 85 males Baseline age: 15–19 years Race: not specified Location: Umea, Sweden |
18 badminton, 44 ice hockey, 23 controls | Method: reported participation in badminton or ice hockey Variables: weight-bearing PA (h/week) |
Follow-up and sustained effects of TB, Hum, LS, FN, and leg aBMD | After adjusting for confounders, badminton players gained significantly more aBMD at all sites compared to both ice hockey players and controls. Ice hockey players gained significantly more aBMD at FN and Hum than at controls. After the end of the active career, ice hockey players and badminton players lost aBMD at a similar rate resulting in still significantly higher aBMD at all sites in badminton players compared to both ice hockey players and controls | |||
| Erlandson et al. 2011 [357] | 4 year prospective case comparison | Sex: 163 males and females Baseline age: 4–7 years Race: 95 % white, 2 % Asian, 3 % other (biracial) Location: Saskatoon, Saskatchewan, Canada |
163 gymnasts, ex-gymnasts, nongymnasts | Method: parental-report questionnaire Variables: current PA score |
Multilevel model comparing TB, FN, and LS BMC | By year 4, recreational and precompetitive gymnasts had 3 % more TB and 7 % more FN BMC than those in other sports when body size, PA, and diet were considered | |||
| Scerpella et al. 2011 [358] | ~12-year prospective case comparison | Sex: 20 females Baseline age: 8–12 years Race: not specified Location: Syracuse, New York, USA |
6 ex-gymnasts, 14 nongymnasts | Method: self-report questionnaire Variables: activity (h/week) |
Follow-up and sustained effect of skull aBMD and BMC; R-UD and 1/3 radius BA, BMC, and aBMD | All R-UD parameters were higher in ex-gymnasts than in nongymnasts (P < 0.02). In contrast, skull aBMD was not associated with gymnastic status. Gymnastic cessation was associated with an abrupt, temporary decrease in 1/3 aBMD, R-UD BMC, and R-UD aBMD. No significant gymnast cessation effects were observed for 1/3 BMC, 1/3 BA, R-UD BA, or skull aBMD | |||
| Erlandson et al. 2012 [359] | 14-year prospective case comparison | Sex: 47 females Baseline age: 8–15 years Race: not specified Location: Saskatoon, Saskatchewan, Canada |
25 gymnasts, 22 controls | Method: self-report questionnaire Variables: past-week PA score |
Follow-up TB, FN, LS BMC, and aBMD | Gymnasts had significantly greater size-adjusted BMC and aBMD compared to nongymnasts at all sites, with the exception of FN aBMD in adulthood. Retired gymnasts had greater size-adjusted TB (13 %), LS (19 %), and FN (13 %) BMC and TB (8 %) and LS (13 %) aBMD compared to nongymnasts. | |||
| Farr et al. 2013 [360] | 2-year prospective follow-up | Sex: 248 females Baseline age: 9–12 years Race: 90 % white, 6 % Asian, 2 % black or African American, 1 % Native American or Alaska Native, 1 % Native Hawaiian or Pacific Islander, and 0.5 % other Location: Tucson, Arizona, USA |
248 | Method: self-report questionnaire Variables: past-year PA score |
Follow-up tibia and femur cortical and trabecular vBMD | PA associated with increases in trabecular vBMD at metaphyseal femur | |||
| Francis et al. 2014 [361] | 10-year prospective follow-up | Sex: 156 males, 170 females Baseline age: 5 years Race: primarily white Location: Iowa, USA |
326 | Method: ActiGraph accelerometer Variables: MVPA min/day (> 2296 ct/min) VPA min/day (> 4011 ct/min) |
Follow-up at ages 13 and 15 LS and TH BMC | Ages 13 and 15 male LS BMC adjusted for baseline LS BMC predicted by age 5 MVPA and VPA | |||
| Janz et al. 2014 [248] | 10-year prospective follow-up | Sex: 217 males, 235 females Baseline age: 5 years Race: primarily white Location: Iowa, USA |
452 | Method: ActiGraph accelerometer Variables: MVPA min/day (>2296 ct/min) VPA min/day (> 4011 ct/min) |
TH and LS BMC at five measurement cycles (age 5, 8, 11, 13, and 15 years). Multilevel model included individual growth curves for each participant | MVPA and VPA added to prediction BMC at all assessed cycles except MVPA LS BMC in females age 5, and males in the 90th percentile for VPA had 8.5 % more hip BMC than males in the 10th percentile for VPA. At age 15, this difference was 2.0 %. Females at age 5 in the 90th percentile for VPA had 6.1 % more hip BMC than those in the 10th percentile for VPA. Age 15 difference was 1.8 %. | |||
| Janz et al. 2014 [248] | 12-year prospective follow-up | Sex: 160 males, 189 females Baseline age: 5 years Race: primarily white Location: Iowa, USA |
349 | Method: ActiGraph accelerometer Variables: MVPA min/day (>2296 ct/min) |
Follow-up (age 17) TB and TH BMC and aBMD | At age 17, girls in high trajectory for MVPA had greater TB BMC, TH BMC, and TH aBMD than in the other trajectories. Boys in the high trajectory for MVPA had greater TB BMC, TH BMC, and TH aBMD than in the other trajectories. | |||
| Cardadeiro et al. 2014 [362] | 1-year prospective follow-up | Sex: 81 males, 96 females Baseline age: 10–12 years Race: primarily white Location: Lisbon area, Portugal |
177 | Method: bone-specific administered questionnaire ActiGraph accelerometer Variables: PA score Sedentary min/day (≤100 ct/min) Light min/day (101–2295 ct/min) Moderate min/day (2296–4011 ct/min) Vigorous min/day (>4012 ct/min) |
Follow-up aBMD PF and subregions | PA measured with questionnaire and accelerometer measured MVPA significant in TR aBMD males. Questionnaire significant in superolateral FN aBMD, inferomedial FN aBMD, and FN aBMD in females | |||
| Lappe et al. 2014 [172] | 6-year prospective follow-up | Sex: 910 males, 833 females Baseline age: 5–19 years Race: nonblack, black Location: Los Angeles, CA; Cincinnati, OH; Omaha, NE; Philadelphia, PA; New York, NY |
1743 | Method: self/parental-report questionnaire (1×/year) Variable: weight-bearing PA (h/week) |
Follow-up and mixed-model analysis %chgBMC for TB, LS, and TH at each TS of maturity | The greatest BMC accrual at all skeletal sites occurred at TS4, with the exception that the greatest % accrual in hip BMC in females was at TS3. Significant PA × TS interactions were only observed in nonblack males and only for TB BMC. All other PA × TS interaction effects were nonsignificant. PA was a significant predictor of chgBMC at all skeletal sites after adjustment for calcium in nonblack males, LS and TH in black males, TB and TH in nonblack females, and at LS in black females. | |||
%chgBMC percent accrual of BMC, 95 % CI 95 % confidence interval, aBMD areal bone mineral density (g/cm2), BA bone area (cm2), BMAD bone mineral apparent density (g/cm2), BMC bone mineral content (g), CON control group, DXA dual-energy x-ray absorptiometry, EX exercise group, FA forearm, FN femoral neck, FS femoral shaft, GRF ground reaction force, GT greater trochanter, Hum humerus, IT intertrochanter, LS lumbar spine, MVPA moderate-through-vigorous-intensity physical activity, NN narrow neck, NS not significant, PA physical activity, PE physical education, PF proximal femur, RA right arm, Rad radius, RCT randomized controlled trial, R-Dis radius, distal, R-Ul radius, ultradistal, SA-BMC size-adjusted bone mineral content, TB total body, T-F tibia–fibula, TH total hip, TR trochanter, TS Tanner stage, U-R ulna-radius, vBMD volumetric bone mineral density (g/cm3), VPA vigorous physical activity, WA Ward’s area
*P < 0.05; **P < 0.01; ***P < 0.001
summarize the articles that were chosen for inclusion in the current review, and these include additional articles located via review articles, meta-analyses, and expert knowledge of the literature.
Results
Nutrition and peak bone mass
Macronutrients
Fat (Table 2)
The search for fat identified no RCTs, 1 prospective study, and 1 cross-sectional study published since 2000, encompassing 163 individuals (Table 2). Data from the prospective study demonstrated that changes in aBMD of the spine in males between ages 16 and 22 years were positively associated with serum levels of arachidonic acid and all omega-3 fatty acids, including DHA [141]. The cross-sectional study by Eriksson et al. [142] showed positive correlations between total body BMC and serum nervonic acid and arachidonic acid as well as negative associations with α-linolenic acid.
Our evidence grade for fat was based on findings from one prospective study with methodological limitations and one cross-sectional study.
Grade: Level of evidence D was assigned for evidence for the benefit of fat on bone.
Protein (Table 3)
The search for protein identified 1 RCT, 5 prospective studies, and 6 cross-sectional studies published since 2000, encompassing 2255 individuals (Table 3). In the one RCT, there was no effect of supplementing 42 g protein over 6 months on changes in tibia trabecular or cortical bone measures at the 4 or 20 % sites, respectively, measured from the distal tibia metaphysis, or on changes in total body BMC [143]. Alexy et al. [144] demonstrated in a cohort of German children that protein intakes over 4 years were positively associated with, and were predictors of, forearm periosteal circumference, cortical area, BMC, and stress–strain index (SSI). The investigators also showed that long-term dietary potential renal acid load (PRAL) was negatively associated with forearm BMC and cortical area. PRAL is increased by sulfur amino acid content of the diet and is decreased by alkaline salts as occurs in plant foods. In the same cohort studied over 4 years, Remer et al. [145] reported that urinary nitrogen (uN) was positively associated with forearm periosteal circumference, cortical area, BMC, and SSI, and urinary PRAL was negatively associated with forearm BMC and cortical area. Protein intakes in males and females between the ages of 2 months and 8 years were positively associated with total body BMC [146]. Using a mixed-longitudinal design, protein intakes were shown to positively predict total body BMC net gain in males and females between ages 8 and 21 years [147]. Moreover, protein intakes in periadolescent females were positively associated with total body BMC and total body BMC net gains, but only in those with calcium intakes >1000 mg/day. Over a period of 5 years, protein intakes in children with low calcium intakes were negatively associated with distal and proximal forearm BMC and total body BMC [148].
Hoppe et al. [149] reported that protein intakes among Danish children were positively related to total body bone area, but not BMC, when adjusted for height, weight, and sex. Differences in arm BMC between twins were partially explained by protein intakes, such that a 1-g difference in protein intake resulted in a 0.4 % difference in arm BMC [150]. Chevalley et al. [151] reported that protein intakes in prepubertal males were positively related to BMC of the radial metaphysis, total radius, femoral neck, femoral diaphysis, and the lumbar spine when controlling for physical activity and calcium intakes. Absolute or adjusted (for body weight, years after menarche, and vertebral area) protein intake from milk, but not from other foods, was positively associated with lumbar spine BMC [152]. In a group of healthy and malnourished Indian children aged 2–3 years, protein intake was positively related to total body BMC and bone area [153]. However, energy-adjusted protein intakes were not significantly associated with forearm geometrical measures in another group of children [154].
Our evidence grade for protein was based on findings from four prospective studies indicating positive findings and one null RCT.
Grade: Level of evidence C was assigned for the benefit of protein on bone.
Micronutrients
Calcium (Tables 4 and 5)
The search for calcium identified 16 RCTs published since 2000, encompassing 3077 individuals (Table 4). In addition, five studies that used both calcium and physical activity interventions were evaluated for main and interaction effects [81, 155–158]. Four observational studies published since 2000, encompassing 2383 individuals, looked at calcium and physical activity interactions on bone (Table 5). When categorized according to the type of calcium intervention, nine studies included supplementation with pills/chews, four used calcium-fortified foods, two used dairy foods, and one used a combination of dairy and pills. Most of the studies included primarily white subjects. A variety of skeletal variables were used as study outcomes. Most studies evaluated the effects of calcium intake on DXA outcomes, including BMC, aBMD, and bone area of the total body, lumbar spine, total hip, femoral neck, intertrochanteric and trochanteric areas of the hip, and distal and ultradistal areas of the forearm. Very few studies reported all possible DXA outcomes, and specific outcomes varied among studies. Three RCTs assessed bone mass and structure using pQCT.
All but one [159] of the nine RCTs using supplement pills found a small, but consistent, positive effect on aBMD and/or BMC accrual as measured by DXA. The benefit to the supplemented group compared to the placebo group ranged from 0.57 to 5.80 %. None of the studies found a significant effect at all (i.e., hip, spine, and radius) of the usual DXA skeletal sites, and the specific sites that benefited varied among the studies. Only three of the RCTs with DXA reported adjusting for body size [160–162], which is important because longitudinal growth confounds interpretation of changes in aBMD and BMC. The difference in height-adjusted BMC accrual between supplemented and placebo groups in these four studies ranged from 0.80 to 4.60 %.
One of the best-designed studies was a single-blind co-twin study of girls aged 8–13 years given calcium carbonate 1200 mg/day or placebo for 24 months [161]. Baseline calcium intake was 786 mg/day (calcium) and 772 mg/day (control; not significant), values considerably lower than the recommended dietary allowance (RDA) (1000 mg/day for ages 4–8 years and 1300 mg/day for ages 9–18 years). Of 64 twin pairs enrolled, 24 pairs completed the study. Compliance with supplementation was 76 % for both groups (calcium and placebo). At the end of study, the calcium group had gained 3.69 % more total body BMC (adjusted for age, height, and weight) than the control group. There were no significant differences in change in BMD at the total hip, spine, or femoral neck. In post hoc analyses, significant differences in gain were seen in total body BMC (2.47 %), hip BMD (1.64 %), and spine BMD (1.64 %) in the calcium group after 12 months of supplementation. Confidence in the findings at 24 months is lessened due to the high rate of attrition (63 %). Another confounder is that the girls studied were peripubertal and thus varied in their estrogen status. At baseline, all subjects were premenarchal, whereas by the end of the study, 13 of the 48 subjects were postmenarchal (five concordant and three discordant pairs.)
Dibba et al. [160] tested the effects of calcium carbonate 1000 mg/day on radius BMC and BMD accrual in a 12-month RCT of prepubertal Gambian children who had low baseline calcium intake (342 mg/day). Supplementation resulted in a higher size-adjusted BMC in the midshaft radius (4.6 ± 0.9 %; P < 0.0001) and in the distal radius (5.5 ± 2.7 %; P = 0.042). This study showed a greater difference in gain between groups than the study by Cameron et al. [161], which supports the premise that children more deficient in a nutrient are likely to have greater benefit from supplementation. Prentice et al. [162] conducted a 13-month RCT of calcium carbonate 1000 mg/day in older adolescent males in Great Britain. Baseline and study calcium intakes were high, even in the placebo group, which was meeting the RDA. Nonetheless, calcium supplementation resulted in an approximately 1 % greater increase in total hip BMC after adjustment for bone area, weight, and height. The calcium intervention also resulted in greater height.
Three RCTs evaluated the effect of both calcium and vitamin D supplement pills on gain in tibial trabecular vBMD as measured by pQCT measurement on the distal tibia. Moyer-Mileur et al. [163] found a significant difference in gain at the 10 % skeletal measurement site on the distal tibia, whereas Greene and Naughton [164] found a significant 5.2 % difference in gain at the 4 % distal tibia site. Both the 10 and 4 % tibia sites represent primarily trabecular bone. Cheng et al. [159] found no effects. Only Greene and Naughton [164] reported adjusting for body size (limb length). However, the vitamin D doses (200 and 400 IU/day) were below the RDA of 600 IU/day, which raises doubt as to whether vitamin D status was optimized in these subjects.
Of the four RCTs using calcium fortification of food or beverages [165–168], all but one [166] found a significant supplement effect on skeletal gain, which ranged from 3.2 to 19.0 %. In the study showing no effect [166], the average dietary calcium intake of the placebo group was 1395 mg/day, which was above the threshold and likely accounts for the lack of effect of a calcium supplement.
Two RCTs were identified that supplemented with dairy foods. One study [169] found that 1000 mg of dairy resulted in a 1.5 % greater gain in spine BMD compared to controls. In the study by Du et al. [170], 10-year-old girls were randomized to one of three groups: group 1 consumed 330 mL/day of ultra-heat-treated (UHT) milk fortified with a calcium salt containing 560-mg calcium, group 2 consumed 330 mL/day of UHT and 200–320 μg of vitamin D, and a control group followed their usual diet. The calcium salt also contained phosphorus and protein, both of which affect bone. Baseline calcium intake was about one third of the RDA among the three groups. After 24 months, groups 1 and 2 gained significantly greater size-adjusted total body BMC than the controls, and group 2 with vitamin D supplementation gained more than group 1 (P = 0.006). Cheng et al. [159] supplemented with both pills and cheese, and the authors found an effect with cheese only.
Three of the four RCTs of calcium and exercise combined [81, 155, 157] found that the combined intervention had a significantly greater effect on bone accrual as assessed by DXA than either exercise or calcium alone. Specker and Binkley [81] also found an interaction effect on cortical thickness and area as measured by pQCT.
Only four observational studies of calcium intake and bone accrual were found [171–174]. Three found no association between calcium intake and accrual, whereas one prospective study [172] found a significant association between calcium intake and spine BMC adjusted for height change only in nonblack girls.
Our evidence grade for calcium was based on positive findings from 90 % of the RCTs using supplement pills, which found a small, biologically, and statistically significant positive effect on aBMD and/or BMC accrual. The bone accrual in the supplemented group compared to the placebo group was largest in children who had the lowest intakes at baseline. This supports the premise of a threshold nutrient such as calcium (i.e., if usual intake of calcium exceeds requirements, then it is unlikely that any benefit of the intervention will be detected). Thus, on the basis of this review, we conclude that dietary intake guidelines for calcium are not being met by all children and adolescents.
Grade: Level of evidence A was assigned for the benefit of calcium on bone.
Vitamin D (Table 6)
The search for vitamin D identified nine publications from 8 RCTs, 1 prospective study, and 3 cross-sectional studies published since 2000, encompassing 2962 individuals (Table 6). Four of the eight RCTs provide evidence for a beneficial effect of vitamin D supplementation on bone accrual. El-Hajj Fuleihan et al. [175] found that Lebanese children receiving weekly doses of 14,000 IU vitamin D3 over 1 year had improved hip BMC and bone area (using conventional DXA) and narrow neck outer diameter and buckling ratio (using the HSA program) [176]. Furthermore, trochanter BMC improved in premenarchal, but not postmenarchal, females [175]. There were no significant effects of supplementation observed in boys. Viljakainen et al. [177] showed that Finnish females assigned to placebo or 200 or 400 IU vitamin D3 over 1 year exhibited no differences in BMC gains at the lumbar spine or femur. A compliance-based analysis was conducted, which included only those subjects with >80 % compliance. After adjusting for bone area, weight gain, and changes in maturation, greater increases in lumbar spine BMC were observed with 400 IU vitamin D3 and in femur BMC with either 200 or 400 IU D3 [177]. The increase in lumbar spine BMC in this study was identified in perimenarchal, but not premenarchal, females. Du et al. [170] demonstrated that the addition of 200–320 IU of vitamin D3 to calcium-fortified milk significantly increased size-adjusted total body BMC over 2 years compared to controls. When analyzing the data by menarchal status, the significant increase in size-adjusted total body BMC was observed only in females that had experienced menarche versus premenarche. Khadilkar et al. [178] found that supplementing vitamin D-deficient girls (girls with mean baseline serum 25(OH)D concentrations 20–25 nmol/L) with quarterly doses of 300,000 IU vitamin D2 along with daily supplementation of 250 mg elemental calcium over 1 year improved adjusted total body BMC and bone area more compared with girls who were supplemented with calcium alone. These positive findings were observed in the compliance-based analyses only and in females within 2 years of starting their menstrual cycle, adjusting for multiple factors including fat-free mass.
Four of the eight RCTs showed no effect of vitamin D supplementation on bone. Ward et al. [179] reported that quarterly supplementation with 150,000 IU vitamin D2 versus placebo in vitamin D-deficient adolescent females did not improve DXA- and pQCT-derived bone outcomes over 1 year, including measures of tibia and radius trabecular or cortical bone. Molgaard et al. [173] reported that supplementation with 200 or 400 IU vitamin D in 10- to 11-year-old females over 1 year had no effect on changes in total body or lumbar spine BMC or in bone area. Similarly, supplementation with either 400 or 800 IU to vitamin D-deficient females over 1 year had no effect on unadjusted total body BMC gains compared to placebo [180]. Finally, supplements containing 200 IU with 1000-mg calcium had no effect on total body, lumbar spine, femoral neck, or total femur BMC gains over 2 years compared to placebo, calcium alone (1000 mg), or a cheese-supplemented group [159].
A prospective study in prepubertal girls conducted over a 5-year period found that as serum 25(OH)D concentrations declined with age, there were significant increases in BMC at multiple skeletal sites [181]. This study showed that serum 25(OH)D did not have a predictive effect on BMC accrual above and beyond that of insulin-like growth factor (IGF)-I [181]. In a cross-sectional study of females aged 10–12 years, those with deficient serum 25(OH)D ≤25 nmol/L had lower cortical vBMD at the distal radius than those with values ≥26 nmol/L, and they also had lower cortical vBMD at the tibial shaft compared to subjects with values between 26 and 40 nmol/L [182]. Moreover, Foo et al. [183] found that 15-year-old Chinese females with vitamin D deficiency (i.e., <25 nmol/L) had lower size-adjusted total body and forearm BMC than females with sufficient values (>50 nmol/L). In a study of 9-year-old Korean children, serum 25(OH)D was positively associated with total body BMC after adjusting for multiple variables, which included physical activity and calcium intake [184].
The evidence grade for vitamin D was based on the point that four of eight RCTs showed improvements in BMC accrual. Furthermore, one of the positive RCTs was well designed, used an intent-to-treat analysis, was adequately powered, and employed a wide range of supplement doses. The evidence grade B reflects the lack of generalizability across the RCTs, which included primarily female subjects with little diversity in population ancestry.
Grade: Level of evidence B was assigned for the benefit of vitamin D on bone.
Micronutrients other than calcium and vitamin D (Table 7)
The search for other micronutrients identified 1 RCT, 2 prospective studies, and 6 cross-sectional studies published since 2000, encompassing 2192 individuals (Table 7). Only one RCT was identified and that was for magnesium [185]. Magnesium supplementation at 300 mg/day for 1 year was significantly associated with a ~3 % increase in overall hip measures of BMC and a borderline significant increase in lumbar spine BMC among white girls. In prospective studies, fluoride was not related to BMC in adjusted models [186–188]. Cross-sectional studies report that a positive association of vitamin C intake and BMC was observed only in boys [189], and positive associations of vitamin C and zinc intakes with bone size and strength were observed in fourth-grade, but not sixth-grade, girls [190]. By contrast, negative associations between high intakes of sodium and phosphorus and total body BMC and bone area in white boys and girls were observed [149]. Iron intake was negatively associated with femoral cortical area [190].
Cross-sectional studies also showed advantages of a biomarker of vitamin K status only in white females [191]. In their study of 245 healthy girls in the USA aged 3–16 years, Kalkwarf et al. [192] reported that better vitamin K status (assessed by plasma phylloquinone and serum percentage of undercarboxylated osteocalcin [%ucOC]) was associated with decreased bone turnover, but it was not associated with baseline BMC. Serum %ucOC was not associated with changes in BMC of the hip, total body, or total body minus the head, but it was surprisingly associated with positive changes in lumbar spine BMC [192]. In contrast with this study, a more recent association study of 223 healthy peripubertal Danish girls found that better vitamin K status was associated with increased total body and lumbar spine BMC, but not with bone turnover [191].
Our evidence grade for other micronutrients was based on one RCT of magnesium supplementation, two prospective studies of fluoride intake, two cross-sectional studies assessing vitamin C and vitamin K intake, and one cross-sectional study assessing intakes of zinc, iron sodium, and phosphorus.
Grade: Level of evidence D was assigned for the benefit of micronutrients other than calcium and vitamin D on bone.
Food patterns (Table 8)
The search for food patterns identified 5 RCTs and 12 observational studies published since 2000, encompassing 6282 individuals (Table 8).
Dairy
Three 2-year RCTs showed increased gains in some bone sites with dairy food consumption [159, 169, 170]. The study by Cheng et al. [159] found an increase with cheese consumption in bone quality assessed by tibia cortical thickness using pQCT in addition to total body BMD, but only in those participants who were at least 50 % compliant. The advantage of trochanter BMC in the study with dairy supplementation disappeared 1 year after cessation [169]. These RCTs were not generalizable because they were conducted only in presumably white girls, except for the study by Du et al. [170] conducted in Asians.
Fiber
One RCT was found and the authors reported a benefit of prebiotic fibers on total body BMC gains in boys and girls over 1 year [193]. One cross-sectional study [194] showed dietary patterns that favored higher bone mass and lower fat mass including higher amounts of dark-green and deep-yellow vegetables and lower amounts of fried foods.
Fruits and vegetables
The five cross-sectional studies identified consistently found some type of benefit to bone with increased and/or high intake of fruits and vegetables [189, 195–198].
Detriment of cola and caffeinated beverages
We identified six studies that examined the effects of carbonated beverages or caffeine-containing beverages and bone accrual during childhood and peak bone mass. Several cross-sectional studies have shown inverse associations between cola or carbonated beverages and bone outcomes in children or young adults. In a population-based, case–control study of children who had experienced upper limb fractures, Ma and Jones [199] found that wrist and forearm fractures were significantly associated with cola drink consumption (odds ratio (OR), 1.39 per unit; 95 % confidence interval (95 % CI), 1.01, 1.91), but not after adjustment for sedentary activities (e.g., television, computer, and video watching). Wyshak [200] found that carbonated beverage consumption was associated with a history of fracture (OR, 3.14; 95 % CI, 1.45, 6.78). The association was strongest for girls reporting higher levels of physical activity (OR, 7.00; 95 % CI, 2.00, 24.45). Similarly, Manias et al. [201] evaluated children with a first-time fracture (n = 50), those who had recurrent fractures (n = 50), and a fracture-free group (n = 50). The recurrent fracture group had lower levels of milk intake and physical activity and higher BMI and carbonated beverage intake than controls; those with one or more fractures had significantly lower total body and lumbar spine BMC and aBMD. A 4-year prospective study of 228 children showed that carbonated beverage consumption increased as milk intake declined, and carbonated beverage intake was negatively associated with strength of the radius (polar SSI) even after adjusting for milk intake [202].
Lower aBMD has been found in children with higher carbonated beverage intakes [194, 201]. McGartland et al. [194] found a significant inverse relationship between total carbonated beverage intake and heel (but not forearm) BMD among girls after adjusting for age, height, weight, pubertal status, social status, alcohol intake, smoking habits, physical activity, liquid milk consumption, and calcium obtained from sources other than liquid milk. These findings suggest that aBMD in girls may be more sensitive to the effects of carbonated beverages compared with boys. McGartland et al. [194] also observed a significant inverse association between carbonated beverage intake and milk intake in both boys and girls.
Remarkably few studies have considered the potential effects of caffeinated beverages on peak bone mass. Caffeine consumption is of concern because it is associated with increased urinary excretion of calcium [203]. In a cross-sectional study of young white women, aged 19–26 years (n = 177), Conlisk and Galuska [204] examined the association of caffeine consumption and femoral neck BMD. Caffeine intake was estimated by self-reported consumption of coffee, decaffeinated coffee, tea, colas, chocolate products, and select medications. After adjustment for potential confounders (height, BMI, age at menarche, calcium intake, protein consumption, alcohol consumption, and tobacco use), caffeine consumption was not significantly associated with aBMD. These findings do not provide support for negative effects of caffeine intake in this age range.
Fruits and vegetables
To our knowledge, there are no long-term prospective studies of vegetarian children with bone outcomes. Few studies have examined the influences of vegetarian dietary patterns on bone, but there is some evidence in adults that adhering to vegan diets is associated with lower bone mass [205] and fractures [206]. It has been hypothesized that following a diet composed primarily of fruits and vegetables would provide a nutrient profile, specifically higher potassium and plant-based proteins, which would favorably influence acid–base balance and bone mass [207]. Alternatively, following a vegetarian diet may exclude certain food groups that contain essential bone-related nutrients such as calcium [208], although a recent large US study using National Health and Nutrition Examination Survey (NHANES) data found no differences in calcium intakes between strict vegetarians and nonvegetarians [209]. In an editorial, Lanham-New [210] takes the position that vegetarianism is not a serious risk factor for osteoporosis. She recognizes that the study of vegetarian dietary patterns and bone is complex because specific patterns of vegetarian diets include or exclude bone-related nutrients and lifestyle factors, serum hormone concentrations, and dietary assessment methods could confound the findings.
Our evidence grade for food patterns was based on 3 RCTs showing a positive benefit of dairy consumption to bone accrual, 1 RCT using mixed chain length fermentable fibers, and 12 observational studies, respectively.
Grade: Level of evidence B was assigned for the benefit of dairy consumption on bone. Level of evidence C was assigned for the benefit of certain types of fiber and fruit and vegetable intake on bone, as well as for a detrimental effect of cola and caffeinated beverages on bone.
Infant nutrition (Table 9)
The search for infant nutrition identified 1 RCT and 10 observational studies published since 2000, encompassing 2715 individuals (Table 9). In the identified RCT, Koo et al. [211] found a positive effect of infant formula enriched with palm olein on bone mineral accretion in healthy term infants compared to the control formula. Of the ten observational studies identified in the search, three compared the effects of duration of breastfeeding [212–214], three assessed later bone outcomes in breast-fed versus formula-fed infants [215–217], two assessed later bone outcomes of breast-fed infants only [218, 219], and two compared breast-fed versus formula-fed versus enriched formula-fed infants [220, 221].
Formula-fed infants had better BMC and BMD in the first 6 months of life compared to breast-fed infants in two of the observational studies [215, 216]; however, breastfeeding was shown to be advantageous in two observational studies assessing later bone outcomes in 8-year-old children [217, 219] and 16-year-old adolescents [218]. Mixed results were obtained for studies testing the duration of breastfeeding in infants who were exclusively breast-fed [212–214]. The addition of palm olein and sn-2 palmitate to infant formula was not shown to be beneficial on later total body BMC outcomes in 4.5- and 10-year-old children [220, 221]. This is contrary to the RCT by Koo et al. [211].
Our evidence grade for infant nutrition was based on the lack of RCTs, inconsistent length of follow-up observational studies, and lack of consistent results across studies.
Grade: Level of evidence D was assigned for the benefit of duration of breastfeeding on bone. Level of evidence D was assigned for the benefit of breastfeeding versus formula feeding on bone. Level of evidence D was assigned for the benefit of enriched formula feeding on bone.
Adolescent special issues
Detriment of DMPA injections and oral contraceptives (Table 10)
The search for contraception identified no RCTs, 8 observational studies, and no cross-sectional studies since 2000, encompassing 1815 individuals (Table 10). Six studies reported null effects of oral contraceptives (OCs) versus a control on bone [222–227] and two studies reported suppression of bone mineral accrual and bone mass acquisition in adolescents [228, 229]. Injections of depot medroxyprogesterone acetate (DMPA) showed a consistent detrimental effect to bone in three studies [225, 226, 230], while one study found null effects [227]. An additional study suggested that the change in body weight due to DMPA injection may override the potential detrimental effect to bone [222].
Grade: Level of evidence B was assigned for the detriment of DMPA injections on bone. Level of evidence D was assigned for the detriment of OCs on bone.
Detriment of alcohol (Table 11)
The search for alcohol identified no RCTs, 3 prospective studies, and 5 cross-sectional studies published since 2000, encompassing 3352 individuals (Table 11). Four studies used peripheral DXA measurements only. There was large variability among studies in the amount of alcohol consumed by study participants and the classification of alcohol intake from ever tried to number of drinks per day. Overall, the reported alcohol consumption by adolescents studied was relatively low. In some studies, adolescents who consumed alcohol were more likely to smoke [231–235], necessitating statistical adjustment for smoking to investigate the independent effects of alcohol intake on bone. The majority of studies found no association between alcohol intake and bone outcomes [232, 233, 235–237]. Among studies reporting a statistically significant association, the direction of the association was inconsistent. Some reported that alcohol intake was associated with lower bone density [231], whereas others reported that alcohol intake was associated with higher bone density [234, 238].
Our evidence grade for alcohol consumption was based on insufficient data to support a hypothesis, owing to no RCTs, low alcohol exposure, and multiple methodological differences among the few studies performed.
Grade: Level of evidence D was assigned for the detriment of alcohol on bone.
Detriment of smoking (Table 12)
The search for smoking identified no RCTs, 6 prospective studies, and 7 cross-sectional studies published since 2000, encompassing 13,955 individuals (Table 12). Six of the studies used peripheral DXA, and two studies examined stress fractures as the bone outcome. There was large variability among studies with respect to extent of smoking in the study participants, both in terms of the proportion who had ever smoked as well as frequency of smoking (e.g., cigarettes per day). Smoking exposure was lowest in young adolescents and increased with age up to young adulthood. Classification of smoking exposure for statistical analyses was also variable across studies (e.g., 1 puff in lifetime versus daily smoking). Some studies reported that adolescents who smoked were more likely than their nonsmoking peers to engage in behaviors that also could negatively impact bone health, namely lower physical activity levels [238, 239], lower dietary calcium intake [239], and greater alcohol use [231–235], making statistical adjustment for these behaviors critical to enable the interpretation of study findings.
Results from studies examining the association between bone density and smoking during adolescence are mixed. Some find significant deficits in bone mass or aBMD at one or more skeletal sites, ranging from −1.8 (not available for all studies) to −6.5 % [232–234, 239, 240], whereas others found no difference in bone according to smoking exposure [231, 235–237]. In the prospective study of adolescent females (aged 13–19 years) by Dorn et al. [233], the effect of smoking on bone accrual became more pronounced as girls got older. Compelling data demonstrating the deleterious effects of smoking on bone come from studies of military recruits. Among male military recruits (aged 18–22 years), Lorentzon et al. [239] found that smoking ≥1 cigarette/day (average 9/day) for an average of 4 years was associated with lower aBMD ranging from −1.8 to 5.0 % depending on the skeletal site. Cortical thickness measured by pQCT was −2.9 to −4.0 % lower in smokers owing to greater endosteal circumference. Eleftheriou et al. [238] found that aBMD at the hip was −4.7 % lower among current smokers compared to never smokers. By contrast, the authors found that ex-smokers had a smaller (−4.3 to −5.0 %) periosteal circumference measured by MRI compared to never smokers, but there were no differences in bone dimensions between current smokers and never smokers. In a study of female military recruits, Lappe et al. [42] found that a history of smoking was associated with an increased risk (OR, 1.34; 95 % CI, 1.05, 1.71) of stress fracture during 8 weeks of basic training. However, years of exercise, which was associated with a reduced risk of stress fracture, was not accounted for in these analyses and effects of smoking may have been overestimated. In a second study of female military recruits, history of smoking was similarly associated (OR, 1.32; 95 % CI, 0.99, 1.75) with risk of stress fracture even when accounting for fitness (running speed) and years of prior exercise [241].
Our evidence grade for smoking was based on multiple well-designed cross-sectional studies.
Grade: Level of evidence C was assigned for the detriment of smoking on bone.
Physical activity and exercise
Effect on bone mass and density (Table 13)
The search for the effects of physical activity on BMC identified 36 RCTs and 20 observational studies published since 2000, encompassing 9942 individuals (Table 13). Eighty-three percent (n = 30) of the RCT studies reported statistically significant (P < 0.05), and many were likely clinically significant (~3 % difference), differences between exercise and control groups at the completion of the intervention. With one exception [242], interventions finding no statistically significant difference between exercise and control groups used similar exercise volume, type, and length as those studies reporting significant effects. Most of the exercise intervention studies of prepubertal, early pubertal, and midpubertal children found increases (~1–6 % difference over 6 months) in the bone mineral of the total body, hip, or lumbar spine. The type of interventions varied but typically ranged from 7 to 24 months in duration, 2–5 sessions per week, 10–60 min per session, and they included sports, games, dance, or high-impact exercises (jumping, hopping). Fewer studies existed for late-pubertal and postpubertal adolescents, and the effects were less dramatic (0.3–1.9 % difference over 6 months), despite a similar intervention dose compared to interventions that focused on younger participants [156, 243]. For example, within the same intervention, one study found skeletal effects in premenarchal but not in postmenarchal females [244].
We reviewed 20 prospective longitudinal studies, with 90 % of these studies (n = 18) reporting statistical differences in bone mass or density between the most physically active children and adolescents in their cohorts and those who were less active. The range in percent difference was wide, although studies that examined youth engaged in organized sports consistently reported greater differences than other study populations. Two studies [54, 245] reported no differences in mass or density between the most active and less active participants. However, these studies used specific self-report measures of physical activity known to have considerable measurement error [246, 247]. By contrast, when using an objective measure of physical activity, the Iowa Bone Development Study demonstrated 10–16 % greater hip BMC and 8 % greater hip aBMD in participants who accumulated the greatest amount of activity from childhood through adolescence (12-year follow-up) [248]. One of the most important of the prospective observational studies, the University of Saskatchewan Paediatric Bone Mineral Accrual Study [3], used a mixed-longitudinal design to evaluate relationships between self-reported general level of physical activity and BMC in a group of healthy Canadian adolescents. The investigators reported that children and adolescents who were physically active at ages 8–15 years had 8–10 % more hip BMC as young adults (aged 23–30 years) compared to less active peers (after controlling for their adult physical activity levels and baseline bone outcomes). This study suggested the possibility of long-term sustained benefits of childhood physical activity on adult BMC [22]. In conclusion, long-term prospective observational studies of heterogeneous cohorts of youth have examined self-selected, everyday physical activity levels and BMC, aBMD, or vBMD. These studies have convincingly and repeatedly shown that participation in high levels of physical activity is associated with greater bone mass accrual compared to less active peers.
Our evidence grade for physical activity and exercise on bone mass and density was based on consistent evidence from many RCTs and observational studies.
Grade: Level of evidence A was assigned for the benefit of physical activity and exercise on bone mass and density.
Effect on bone structural outcomes (Table 14)
Table 14.
Physical activity and exercise on bone structure in children and adolescents
| Reference | Study description | Population description | Number of subjects | Intervention | End points | Results | ||
|---|---|---|---|---|---|---|---|---|
| RCTs | ||||||||
| Heinonen et al. 2000 [244] | 9-month step aerobics/ jumping intervention Randomized by school |
Sex: female Age: 10–15 years Race: not specified Location: Tampere, Finland |
126 | Frequency: 2×/week Intensity: progressive Time: 50 min (10-min warm-up, 15-min nonimpact aerobic exercises, 20-min high-impact/jump training, 5-min cool down) Type: both-leg jumps at floor level, both-leg box jumps, one-leg box jumps Control: normal PA |
pQCT Significant None NS Tibia CoD, CoA, BSI |
At the tibial midshaft, the intergroup differences (CoD, CoA, and BSI) were not significant | ||
| Petit et al. 2002 [73] | 7-month jumping intervention Randomized by school |
Sex: female Age: 9–12 years Race: multiethnic population of city (~34 % Hong Kong Chinese and 57 % white) reflected in cohort Location: Richmond, British Columbia |
177 | Frequency: 3×/week (2× PE, 1 classroom) Intensity: progressed over school year by increasing number of jumps per station (from 10 to 20) and height (from 10 to 50 cm) Time: 10–12 min (5 circuits, ~1.5–2 min each) Type: jumping exercises—jumping jacks, lung jumps, hopping, jumping over various obstacles, drop jumps from platform Control: stretching |
pQCT | Change in early pubertal girls, mean (95 % CI) | ||
| Significant | EX | CON | ||||||
| NN CSA | 0.060 (0.043, 0.077)* | 0.035 (0.021, 0.049) | ||||||
| NN Z | 0.145 (0.118, 0.0172)* | 0.106 (0.084, 0.129) | ||||||
| NN CT | 0.012 (0.008, 0.016)* | 0.007 (0.004, 0.010) | ||||||
| IT ED | 0.092 (0.064, 0.120)* | 0.138 (0.114, 0.161) | ||||||
| NS | There was no difference in change for bone structure in the PRE girls. The more mature girls (EARLY) in the EX group showed significantly greater gains in CSA and reduced endosteal expansion. Changes in SPW did not differ. Structural changes improved Z at the NN (4.0 %), but not at the IT region. There were no differences at the primarily cortical FS. | |||||||
| NN length, SPW, ED | ||||||||
| IT CSA, SPW, Z | ||||||||
| FS CSA, SPW, Z, ED, CT | ||||||||
| Specker and Binkley 2003 [81] | 1-year randomized, placebo-controlled, partially blinded trial of PA and calcium supplementation | Sex: male and female Age: 3–5 years Race: 94 % white, 6 % other Location: South Dakota |
178 | Frequency: 5 days/week Intensity: not specified Time: 30 min/day (5-min warm-up, 20-min activity, 5-min cool down) Type: hopping, jumping, skipping activities (17 different weekly programs) Control: 30 min/day of activities to keep them sitting quietly |
pQCT | Exercise group had greater PCirc and ECirc than control group (P < 0.05). | ||
| Significant | ||||||||
| PCirc | ||||||||
| ECirc | ||||||||
| NS | ||||||||
| CA, CT | ||||||||
| MacKelvie et al. 2004 [295] | 20 month jumping intervention Randomized by school |
Sex: male Age: 8.8–12.1 years Race: multiethnic population of city (~34 % Hong Kong Chinese and 57 % North American/Western European Caucasian, 5 % Southeast Asian, and 4 % other or mixed ethnicity) reflected in cohort Location: Richmond, British Columbia |
64 | Frequency: 3×/week (2× PE, 1 classroom) Intensity: progressed over school year by increasing number of jumps per station (from 10 to 20) and height (from 10 to 50 cm Time: 10–12 min (5 circuits, ~1.5–2 min each) Type: jumping exercises- jumping jacks, lung jumps, hopping, jumping over various obstacles, drop jumps from platform Control: stretching |
HSA | Change, mean (95 % CI) | ||
| Significant | EX | CON | ||||||
| NN CSMI | 0.23 (0.20, 0.27)* | 0.17 (0.14, 0.20) | ||||||
| NN Z | 0.13 (0.11, 0.15)* | 0.09 (0.072, 0.11) | ||||||
| NS | At the NN region, EX boys had significantly greater changes in Z (7.5 %). Changes at the IT and FS regions were not significantly different between groups. | |||||||
| NN length, CSA, PW, EW, CT | ||||||||
| IT CSMI, CSA, PW, Z, ED, CT | ||||||||
| FS CSA, PW, CSMI, Z, EW, CT | ||||||||
| McKay et al. 2005 [297] | 8-month jumping intervention Control group used from previous study |
Sex: male and female Age: 8.9 to 11.0 years Race: 38 % Caucasian, 48 % Asian, 15 % other (including mixed ethnic, black, and South Asian) Location: Richmond, British Columbia |
124 | Frequency: 3×/day Intensity: 5× body wt, maximum rate of force was > 400 body wt/s (independent sample of 70 boys and girls) Time: 3 min/day Type: 10 counter movement jumps (two-foot take off, clutch knees, two-foot landing) Control: not specified, from previous study |
HSA | Change in bone structural parameters did not differ between groups. | ||
| Significant | ||||||||
| None | ||||||||
| NS | ||||||||
| NN and FS BMD, CSA, SW, Z, ED, CT | ||||||||
| Macdonald et al. 2007 [25] | 16-month PA and jumping intervention Randomized by school |
Sex: male and female Age: 10.2 ± 0.6 years Race: 53 % Asian (Hong Kong or China, India Philippines, Vietnam, Korea, or Taiwan), 35 % Caucasian (North America or Europe), 12 % mixed or other ethnic origins Location: Richmond and Vancouver, British Columbia |
410 | Frequency: Classroom Action: 5 days/week; Bounce at the Bell: 5 days/week PA + 3×/day, 4 days/week jumping Intensity: not specified Time: Classroom Action: 15 min of PA; Bounce at the Bell: 15 min of PA + short bouts of high-impact jumping Type: Classroom Action: skipping, dancing, playground circuits and simple resistance exercises with bands; Bounce at the Bell: Classroom Action + short bouts high-impact jumping Control: regular PE |
pQCT | Change in bone structural parameters did not differ between groups. | ||
| Significant | ||||||||
| None | ||||||||
| NS | ||||||||
| Distal and midshaft BSI, ToA, ToD | ||||||||
| Linden et al. 2007 [298] | 1 year expanded PE intervention Randomized by school |
Sex: Male Age: 6.7–9.0 years Race: Caucasian (except 1 boy adopted from Colombia) Location: Malmö, Sweden |
138 | Frequency: 5 days/week Intensity: varied Time: 40 min/day Type: normal PE curriculum (running, jumping, climbing ropes, variety of ball games). Activities varied to reduce boredom. No specific osteogenic training program was added. Control: normal PE curriculum, duration within normal limits (1–2 sessions/week, in total 60 min/week) |
HSA | Change in bone structural parameters did not differ between groups. | ||
| Significant | ||||||||
| None | ||||||||
| NS | ||||||||
| FN CSMI, Z, CSA | ||||||||
| Macdonald et al. 2008 [299] | 8-month PA and jumping intervention Randomized |
Sex: male and female Age: 9–11 years Race: 53 % Asian (both parents or all four grandparents born in Hong Kong or China, India, Philippines, Vietnam, Korea, or Taiwan. 35 % Caucasian (parents born in North America or Europe). 12 % children mixed ethnicity or other ethnic origins Location: Vancouver and Richmond, British Columbia |
410 | Frequency: Classroom Action: 5 days/week; Bounce at the Bell: 5 days/week PA + 3×/day, 4 days/week jumping Intensity: not specified Time: Classroom Action: 15 min PA; Bounce at the Bell: 15 min PA + short bouts high-impact jumping Type: Classroom Action: skipping, dancing, playground circuits, simple resistance exercises with bands; Bounce at the Bell: Classroom Action + short bouts high-impact jumping. Phase I included 5 two-foot landing jumps (or 10 one-foot landing jumps) at each session. Phase II jumps were increased each month of the school year until a maximum of 36 jumps/day was reached. Control: Usual PE |
HSA | Change in bone structural parameters did not differ between groups. | ||
| Significant | ||||||||
| None | ||||||||
| NS | ||||||||
| NN Z, CSA, SPW | ||||||||
| Alwis et al. 2008 [300] | 1-year expanded PE intervention Randomized by school |
Sex: female Age: 6.5–8.9 years Race: Caucasian Location: Malmö, Sweden |
103 | Frequency: 5 days/week Intensity: varied Time: 40 min/day Type: normal PE curriculum (running, jumping, climbing ropes, variety of ball games). Activities varied to reduce boredom. No specific osteogenic training program was added. Control: normal PE curriculum, duration within normal limits (1–2 sessions/week, in total 60 min/week) |
HSA | Change in bone structural parameters did not differ between groups. | ||
| Significant | ||||||||
| None | ||||||||
| NS | ||||||||
| FN length, PW, CSA, Z, CSMI, EW, CT | ||||||||
| Alwis et al. 2008 [300] | 2-year expanded PE intervention Randomized by school |
Sex: male Age: 6.7–9.0 years Race: Caucasian Location: Malmö, Sweden |
137 | Frequency: 5 days/week Intensity: varied Time: 40 min/day Type: normal PE curriculum (running, jumping, climbing ropes, variety of ball games). Activities varied to reduce boredom. No specific osteogenic training program was added. Control: normal PE curriculum, duration within normal limits (1–2 sessions/week, in total 60 min/week) |
HSA | Change in bone structural parameters did not differ between groups. | ||
| Significant | ||||||||
| None | ||||||||
| NS | ||||||||
| FN CSA, Z, CSMI | ||||||||
| Alwis et al. 2008 [300] | 2-year expanded PE intervention Randomized by school |
Sex: female Age: 6.8–8.9 years Race: Caucasian Location: Malmö, Sweden |
291 | Frequency: 2×/week (PE); 3×/day (activity breaks); 1×/day (home activity) Intensity: not specified Time: 45 min/session (PE); 2–5 min/session (activity breaks); 10 min/day (home activity) Type: 2 PE lessons (plus 3 regular PE classes) taught mostly outdoors by PE teachers. All five sessions included jumping activities such as hopping, jumping up and down stairs, rope skipping, etc. During academic lessons, 3–5 activity breaks comprised motor skill tasks such as jumping around on one leg, balancing on one leg, power games, or coordinative tasks were introduced every day. Daily home activity included aerobic, strength, or motor skill tasks such as tooth brushing while standing on one leg, jumping up and down the stairs, and rope jumping. Control: participated in regular PE classes 3×/week |
HSA | Change in bone structural parameters did not differ between groups. | ||
| Significant | ||||||||
| None | ||||||||
| NS | ||||||||
| FN CSA, PW, Z, CSMI | ||||||||
| Weeks et al. 2008 [296] | 8-month jumping intervention Randomized |
Sex: male and female Age: boys 13.8 years (0.4); girls 13.7 years (0.5) Race: not specified Location: Gold Coast, Australia |
81 | Frequency: 2×/week Intensity: 1–3 Hz at a height of 0.2–0.4 m Time: 10 min (~300 jumps) Type: varied, but included- jumps, hops, tuck jumps, jump squats, stride jumps, star jumps, lunges, side lunges, and skipping. Occasionally supplemented with upper body strengthening activities, including push-ups and exercises with resistive latex bands Control: usual PE warm-up and stretching |
DXA | Percent change, mean | ||
| Significant | EX | CON | ||||||
| LS IBS | 17.9* | 14.4 | ||||||
| NS | LS IBS improved more for EX participants than for CON. No other differences in 8-month change in parameters of bone strength reached significance. | |||||||
| FN, LS BMAD | ||||||||
| FN CSMI | ||||||||
| Greene et al. 2009 [301] | 28-week drop-landing intervention Randomized |
Sex: female Age: 6–10 years Race: not listed Location: Sydney, Australia |
39 | Frequency: 3×/week Intensity: 14-cm height drop for low drop, 28-cm height drop for high drop (GRF 2–4× body wt); 10 sets of 5 steps per set; 50 steps per session Time: 15 min/day Type: single leg drop-landing exercises with nondominant leg serving as the training leg (dominant leg served as within-participant untrained control) Control: normal activity |
pQCT | Change in bone structural parameters did not differ between groups. | ||
| Significant | ||||||||
| None | ||||||||
| NS | ||||||||
| Cort, Medullary, and Total BA at Proximal, Mid, Distal | ||||||||
| Macdonald et al. 2009 [250] | 16-month PA and jumping intervention Randomized |
Sex: male Age: 10.2 ± 0.6 years Race: 52 % Asian (Hong Kong or China, India, Philippines, Vietnam, Korea, or Taiwan), 35 % Caucasian (North America or Europe), 12 % mixed or other ethnic origins Location: Richmond and Vancouver, British Columbia |
205 | Frequency: Classroom Action: 5 days/week; Bounce at the Bell: 5 days/week PA + 3×/day, 4 days/week jumping Intensity: not specified Time: Classroom Action: 15 min PA; Bounce at the Bell: 15 min PA + short bouts high-impact jumping Type: Classroom Action: skipping, dancing, playground circuits, simple resistance exercises with bands; Bounce at the Bell: Classroom Action + short bouts high-impact jumping. Phase I included 5 two-foot landing jumps (or 10 one-foot landing jumps) at each session. Phase II jumps were increased each month of the school year until a maximum of 36 jumps/day was reached. Control: Usual PE |
pQCT | Difference, mean (95 % CI) | ||
| Significant | ||||||||
| I max | 338.6 (16.4, 660.9)* | |||||||
| NS | The EX boys had a 3 % greater gain in I max than CON boys. No other bone structural parameters differed between groups. | |||||||
| I min, I max/I min, CoA- Ant, CoA- Med, CoA- Post, CoA- Lat, CTh- Ant, CTh- Med, CTh- Post, CTh- Lat | ||||||||
| Lofgren et al. 2012 [28] | 4-year expanded PE intervention Randomized by school |
Sex: male and female Age: 6.5–8.7 years Race: Caucasian Location: Malmö, Sweden |
221 | Frequency: 5 days/week Intensity: varied Time: 40 min/day Type: normal PE curriculum (running, jumping, climbing ropes, variety of ball games). Activities varied to reduce boredom. No specific osteogenic training program was added Control: normal PE curriculum, duration within normal limits (1–2 sessions/week, in total 60 min/week) |
HSA | Change, mean (95 % CI) | ||
| Male | Female | |||||||
| EX | CON | |||||||
| FN CSA | 0.11 (0.10, 0.13)* | 0.09 (0.07, 0.11) | ||||||
| FN Z | 0.06 (0.05, 0.06)* | 0.05 (0.04, 0.06) | ||||||
| FN CSMI | 0.10 (0.08, 0.11)* | 0.08 (0.07, 0.09) | ||||||
| The EX girls had significantly greater gain in all HSA outcomes than CON girls. No differences were seen for boys. | ||||||||
| Anliker et al. 2012 [302] | 9-month jumping intervention Randomized by school |
Sex: male and female Age: 8–12 years Race: not listed Location: Lucerne, Switzerland |
45 | Frequency: 2×/week Intensity: intensity increased weekly. Number of jumps increased over time from ~60 to ~150 per session Time: 10 min Type: Jumping and sprinting exercises, including two- and one-legged hopping, drop jumps, side to side jumps, jumping jacks, jumps and landings from a podium, jumps over barriers and short multidirectional sprints Control: not specified |
pQCT | Change in bone structural parameters did not differ between groups. | ||
| Significant | ||||||||
| None | ||||||||
| NS | ||||||||
| Tibia 4 % vBMC, vBMDtr, vBMDtot, BAtb, BAtot | ||||||||
| Detter et al. 2014 [303] | 6-year expanded PE intervention Randomized by school |
Sex: male and female Age: 6–8 years Race: Caucasian Location: Malmö, Sweden |
133 | Frequency: 5 days/week Intensity: varied Time: 40 min/day Type: normal PE curriculum (running, jumping, climbing ropes, variety of ball games). Activities varied to reduce boredom. No specific osteogenic training program was added Control: normal PE curriculum, duration within normal limits (1–2 sessions/week, in total 60 min/week) |
Cross-sectional pQCT | Change in bone structural parameters did not differ between groups. | ||
| Significant | ||||||||
| None | ||||||||
| NS | ||||||||
| Tibia 4 %, Radius 4 % | ||||||||
| TR vBMD | ||||||||
| Tibia 38 %, Radius 66 % | ||||||||
| Cort vBMD | ||||||||
| Cort BMC | ||||||||
| Cort BA | ||||||||
| Cort Th | ||||||||
| CSA | ||||||||
| Polar SSI | ||||||||
| Observational studies | ||||||||
| Reference | Study description | Population description | N | Exposure | End points | Results | ||
| Forwood et al. 2006 [363] | 7-year prospective follow-up | Sex: 109 males, 121 females Baseline age: 8–15 years Race: white Location: Saskatoon, Saskatchewan, Canada |
230 | Method: self-report questionnaire (3×/year for 3 years, 2×/year after) Variable: general level of PA for year (1–7) |
Multilevel models for FN Z, CSA, and SPW (HSA) | MVPA positively associated with FN Z and FN CSA males and females. A male with PA score of 1 had 0.0972 cm2 less CSA than a male with PA score of 5. Females with PA score of 1 had 0.0588 cm2 less CSA than females with score of 5. Males with PA score of 1 had 0.0615 cm2 less Z than males with PA score of 5. Females with PA score of 1 had 0.0355 cm2 less Z than females with score of 5. | ||
| Janz et al. 2007 [364] | 6-year prospective follow-up | Sex: 212 males, 233 females Baseline age: 5 years Race: primarily white Location: Iowa, USA |
445 | Method: ActiGraph accelerometer Variable: MVPA min/day (>3000 ct/min) |
Multilevel models for FN Z and CSA (HSA) | MVPA positively associated with Z and CSA males and females. When adjusted, LM only significant males | ||
| 40-min MVPA predicted 3–5 % greater CSA and Z compared to 10 min/day MVPA | ||||||||
| When CSA and Z adjusted for LM, associations attenuated in males but remain significant. Associations become insignificant in females. | ||||||||
| Farr et al. 2013 [360] | 2-year prospective follow-up | Sex: 248 females Baseline age: 9–12 years Race: 90 % white, 6 % Asian, 2 % black or African American, 0.5 % Native American or Alaska Native, 1 % Native Hawaiian or Pacific Islander, 0.5 % other Location: Tucson, Arizona, USA |
248 | Method: self-report questionnaire Variable: past-year PA score |
Follow-up tibia and distal femur BSI | PA associated with increases in distal femur BSI | ||
| Gruodyte-Raciene et al. 2013 [365] | 4-year prospective follow-up | Sex: 81 males, 84 females Baseline age: 4–10 years Race: 96 % white, 2 % Asian, 2 % other Locations: Saskatoon, Saskatchewan, Canada |
165 92 gymnasts, 73 nongymnasts |
Method: parental-report questionnaire Variable: PA score 1–2 h/week gymnastics exposure |
Yearly trajectories of PF CSA and Z (HSA) | When compared to nongymnasts, gymnasts 6 % greater NN CSA, 7 % greater NN Z, 5 % greater IT CSA, 6 % greater IT Z, and 3 % S CSA | ||
| Janz et al. 2014 [248] | 12-year prospective follow-up | Sex: 160 males, 189 females Baseline age: 5 years Race: primarily white Location: Iowa, USA |
349 | Method: ActiGraph accelerometer Variable: MVPA min/day (≥2296 ct/min) |
Follow-up (age 17) FN Z and CSA Tibia BSI Tibia polar moment of inertia |
Participants in high trajectory for MVPA had greater geometric outcome measures (Z: females 21.0 %, males 9.4 %; CSA: females 11.8 %, males 10.0 %; BSI: females 19.0 %, males 12.8 %; polar moment of inertia: females 38 %, males 8.4 %) at age 17 compared to other trajectories. | ||
| Jackowski et al. 2014 [251] | ~15-year prospective follow-up | Sex: 55 males, 49 females Baseline age: 8–15 years Race: primarily white Location: Saskatoon, Saskatchewan, Canada |
104 | Method: self-report questionnaires Variable: average activity score |
Follow-up (age 23–30) PF CSA and Z (HSA) | Around time of PHV, active adolescents had 8–12 % greater CSA and 9–12 % greater Z than inactive peers at PF. When adult CSA and Z were adjusted for height, weight, adolescent bone geometry, and sex, those active during adolescence maintained 5–7 % benefit in CSA and 6–8 % benefit in Z in adulthood compared to inactive. In young adulthood, males and females who were physically active as adolescents had greater bone geometric measures. | ||
| Duckham et al. 2014 [252] | ~18-year prospective follow-up | Sex: 49 males, 73 females Baseline age: 8–15 years Race: primarily white Location: Saskatoon, Saskatchewan, Canada |
122 | Method: self-report questionnaire Variable: general PA score |
Follow-up (age 24–34) tibia and radius BA, BSI, SSIp, CoC, CoA, CoD, ToA, ToD, TrC, TrD, and BSIc (pQCT) | In young adulthood, males who were physically active as adolescents had 13 % greater SSIp and 10 % greater ToA at tibia. Females had 10 % larger CoA, 12 % larger CoC, and 3 % larger TrC at tibia. | ||
| Cardadeiro et al. 2014 [362] | 1-year prospective follow-up | Sex: 81 males, 96 females Baseline age: 10–12 years Race: primarily white Location: Lisbon area, Portugal |
177 | Method: bone-specific administered questionnaire ActiGraph accelerometer Variables: PA score Sedentary min/day (≤100 ct/min) Light min/day (101–2295 ct/min) Moderate min/day (2296–4011 ct/min) Vigorous min/day (>4012 ct/min) |
BMD distribution via three aBMD ratios: FN/PF, IM/SL, and TR/PF Geometric measures of the pelvis—IAD and PF ALA |
Questionnaire significant in FN/PF ratio model in males | ||
95 % CI 95 % confidence interval, aBMD areal bone mineral density (g/cm2), ALA abductor lever arm, Ant anterior, BA bone area (cm2), BMAD bone mineral apparent density (g/cm2), BMC bone mineral content (g), BMD bone mineral density (g/cm2), BSI bone strength index (mg2/cm4), BSI c bone strength in compression, CA cortical area (mm2), CoA cortical bone area (mm2), CoC cortical content (mg/mm), CoD cortical density (mg/cm3), CON control group, Cort cortical, CSA cross-sectional area (cm2), CSMI cross-sectional moment of inertia, CT cortical thickness (mm), ct cortical, ECirc (mm) endosteal circumference, ED endosteal diameter (cm), endo endosteal, EW endosteal width, EX exercise group, FN femoral neck, FS femoral shaft, HSA hip structure analysis, IAD interacetabular distance, IBS index of bone structural strength, IM inferomedial, I max maximum moment of area, I min minimum moment of area, IT intertrochanter, Lat lateral, LM lean mass, LS lumbar spine, Med medial, MVPA moderate-through-vigorous-intensity physical activity, NN narrow neck, NS not significant, PA physical activity, PCirc periosteal circumference (mm), PE physical education, peri periosteal, PF proximal femur, PHV peak height velocity, Post posterior, pQCT peripheral quantitative computed tomography, PW periosteal width, RCT randomized controlled trial, SL superolateral, SPW shaft periosteal width (cm), SSI strength strain index, SSIp density-weighted polar section modulus (mm3), SW subperiosteal width (cm), tb trabecular, Th thickness, ToA total area (mm2), ToD total BMD (mg/cm3), tot total, TR trabecular, TrC trabecular content, vBMC volumetric BMC (g/cm), vBMD volumetric bone mineral density (g/cm3), Z section modulus (cm3)
*P < 0.05; **P < 0.01; ***P < 0.001
The search for the effects of physical activity on bone structure/geometry identified 17 RCTs and 8 observational studies published since 2000, encompassing 4722 individuals (Table 14). Slightly more than one third (n = 6) reported statistically significant effects of exercise on bone structural outcomes. However, of the 11 studies that reported no statistical differences between exercisers and controls, six reports were from the same study (the Malmö Pediatric Osteoporosis Prevention Study). This study was designed to evaluate whether increasing time in physical activity in a cohort could be used as a population-based prevention strategy to improve bone outcomes. Prepubertal children were randomized by schools into a 5-day/week, 40-min/day physical education curriculum or a 1- to 2-day/week, 60-min/week curriculum. The content of the physical education curricula did not differ and specific osteogenic exercise was not prescribed. By contrast, in a 1-year RCT in young children (aged 3–5 years) randomized at the individual level, Specker and Binkley [81] used research staff to deliver (presumably) osteogenic exercise (gross motor skills such as hopping, jumping, and skipping) and reported that gross motor skill exercise increased periosteal and endosteal circumferences at the 20 % site of the distal tibia compared to fine motor skill exercise. The effect of gross motor exercise (2 % difference) persisted 1 year after follow-up; however, the intervention group was also more physically active at least 6 months after the intervention (raising the possibility that the sustained effect was due to continued high levels of physical activity) [249]. Using a 7-month, 3-day/week, ~12-min/day jumping protocol, researchers with the University of British Columbia Centre for Hip Health and Mobility [73] reported structural changes at the hip in early pubertal girls (but not prepubertal girls) compared to controls who stretched. The difference in section modulus, a measure of the strength of bone during bending, was 4 %. A similar University of British Columbia project by Macdonald et al. [250] used a 16-month, 5-day/wk, ~15-min/day jumping protocol and compared this intervention to usual physical education (which the intervention participants also received). A significant difference of 3 % greater tibia midshaft tibia cross-sectional moment of inertia (CSMI) in boys was reported. Changes in CSMI suggest a change in cross-sectional geometry due to increased periosteal apposition in one of the planes. However, other structural differences were not statistically significant in the study by Macdonald et al. [250].
We identified eight prospective observational studies that examined associations between physical activity and whole bone structure. All studies (100 %) found statistically significant, and likely clinically significant, differences between the most active and less active cohort members. The University of Saskatchewan Pediatric Bone Mineral Accrual Study found an 8–12 % greater CSA and section modulus of the proximal femur in young adults who were active as adolescents (compared to peers who were less active as adolescents) [251]. In the same cohort, Duckham et al. [252] reported 13 % greater polar SSI and 10 % total bone CSA of the tibia in young adults who were active as adolescents compared to less active peers during adolescence. In females, differences of 10 % greater cortical CSA and 12 % cortical content of the tibia were found. CSA and section modulus of the femoral neck as well as measures of tibial compressive and torsional strength have also been associated with physical activity in the Iowa Bone Development Study cohort. On average, the investigators reported a 14 % difference in various measures between cohort members who were most active during a 12-year follow-up compared to those who were less active [248].
Our evidence grade for physical activity and exercise on bone structure was based on semi-consistent evidence from many RCTs and observational studies.
Grade: Level of evidence B was assigned for the benefit of physical activity and exercise on bone structure.
Discussion
In this scientific statement, we updated a former effort published in 2000 to summarize the lifestyle choices that influence development of peak bone mass [1]. Unlike the earlier report, our review used a systematic approach to search predictors of bone mass from publications since 2000. We also considered our knowledge of physiological functions and biology of growth for areas for which a systematic review was not possible. Bone is a living tissue and as such requires all essential nutrients for growth and maintenance. The bony mineral tissue is composed of hydroxyapatite, a calcium-phosphate compound, with magnesium and trace amounts of other minerals. The connective tissue is composed primarily of the protein, collagen. The role of many micronutrients in bone is to assist in connective tissue synthesis and maturation. Iron, zinc, magnesium, copper, manganese, and vitamin K are cofactors in enzymes responsible for bone metabolism, collagen synthesis, and cross-linking. Vitamin D is metabolized to a steroid-like hormone that increases calcium absorption through a saturable, facilitated diffusion pathway. Mechanical loading from physical activity is essential to stimulate bone modeling to provide the stimulus necessary to develop a strong skeleton to support growth and development. In children and adolescents, the important focus is on bone accrual, with careful monitoring of growth parameters. Unfortunately, in contrast with adults, children and adolescents have not been the focus of research in many studies relating lifestyle factors to bone density or quality.
Grade A evidence
Both physical activity and calcium intake had strong and abundant evidence to be assigned a grade A level of evidence. This level of evidence is not often attained and merits priority action for public health efforts. Regrettably, calcium intake and physical activity are not achieved in recommended levels by our youth. A large difference in the nature of the evidence between physical activity and calcium intake is apparent. The evidence for physical activity and bone mass and geometry is a global approach, whereas the evidence for calcium intake and bone mass is a reductionist approach. The research available for physical activity does not examine the effects of specific types of exercise and few studies examine the dose loading effects of any one type of exercise. Therefore, we conclude that physical activity is important for growing bone, but we do not fully understand the characteristics of physical activity that impact bone such as mode, frequency, intensity, and duration. On the other hand, studies of the effects of diet on bone usually look at a single nutrient effect; there is much less evidence for the effects of diet quality as a whole. There is opportunity for researchers in both fields to consider the approaches of the other field.
Macronutrients
Fat
There was significant interest in dietary fat and bone metabolism in the decade preceding the 2000 review by Heaney et al. [1] that centered around ω-3 and ω-6 fatty acids and biomarkers of inflammation, primarily in animal models. Since 2000, the work has continued to examine the long-chain ω-3 fatty acids, DHA and eicosapentaenoic acid. The majority of studies have been conducted in adults, and the findings are equivocal with respect to improvements in bone mass [253]. Only one RCT was identified in children, but it was not included in this review due to the short 16-week duration of the intervention [254]. Prospective studies and RCTs in children and adolescents are lacking, and it is premature at this time to draw conclusions regarding the influences of dietary fat on bone during growth.
Protein
During pubertal growth, BMC accrual is markedly influenced by increasing IGF-I [181], and IGF-I is impacted by energy and protein intakes. We considered studies addressing both dietary protein intakes as well as PRAL. Prior to the 2000 review on peak bone mass by Heaney et al. [1], the interest in dietary protein and bone centered on calcium/protein ratios and calcium retention in adults, although the findings of several protein and bone cross-sectional studies in adults were mixed. Other dietary factors are also of interest including the effect of specific dietary proteins with higher sulfur-containing amino acids, which increase PRAL and may lead to lower bone quality. Much of what is known regarding dietary protein and bone quality emanates from adult studies, with limited work in children and adolescents. One short (6 months) RCT in adolescents [143] showed no benefits to material or geometrical properties of bone and was not generalizable because it included only late adolescents and young adults aged 18–25 years. The majority of prospective [144–147] and cross-sectional [149–151, 153] studies support a positive relationship between protein intake and bone. The Dortmund Nutritional and Anthropometric Longitudinally Designed (DONALD) Study [144, 145] demonstrated that protein intake was positively, and PRAL was negatively, associated with the geometrical properties of the forearm in a stepwise multiple regression model. Using biomarker data from that same cohort, long-term protein intake estimated by uN excretion and urinary PRAL were positively and negatively associated with forearm cortical BMC and area, respectively, when adjusting for age, sex, pubertal stage, forearm muscle area, forearm length, and urinary calcium. To further support a positive effect of dietary protein on bone growth, protein intake over approximately 8 years explained total body BMC net gain in the University of Saskatchewan Pediatric Bone Mineral Accrual Study [147]. In a multivariate regression model, long-term protein intake (over 2 years) positively predicted total body BMC. Collectively, the prospective studies lend support for a positive effect of protein on bone in growing children.
Only one prospective study showed negative relationships between dietary protein and bone [148]. The authors suggested that the negative relationships might have been due to low calcium intakes among the children. Consistent with this notion, Vatanparast et al. [147] reported that the positive effect of dietary protein on bone mass is most evident in those consuming adequate calcium (>1000 mg/day). Higher dietary protein accompanied by low calcium intakes (i.e., lower calcium/protein ratio) could lead to increased urinary calcium excretion [255] and lower bone mass; however, RCTs are needed to prove this assumption.
Micronutrients
Calcium
Storage of calcium in bone serves as a functional reserve to offset dietary shortages of calcium and is tapped when needed to maintain homeostasis. More than 99 % of the body’s calcium is in the skeleton as a consistent proportion of bone mineral. The calcium reserve is very large relative to the cellular and extracellular metabolic pools of calcium; thus, dietary insufficiency rarely impairs calcium-dependent biochemical functions. However, long-term deficiency depletes the reserve and subsequently decreases bone mass and bone strength.
Because the human skeleton contains only about 2–3 % of the total adult body calcium at birth, the dietary requirements for calcium during the first 20–30 years of life are determined primarily by skeletal growth. Extreme calcium deficiency during growth can cause rickets [256, 257]. However, even moderate deficiency has deleterious effects on the skeleton, both short term and long term.
Balance studies are useful to show the effect of a nutrient in an otherwise controlled environment because the diet is strictly controlled. These studies have shown that calcium is a threshold nutrient, implying that calcium retention increases with calcium intake until a plateau is reached. Balance studies, rather than bone density or other skeletal measures, have been used to demonstrate this phenomenon because finding the threshold requires a range of intakes, which bracket the threshold intake. This is possible in balance studies that are sufficiently short to manage controlled diets until steady state is achieved. During peak bone mass accrual, there are racial and sex differences in the plateau calcium intake and the peak maximal retention in adolescents. Black girls have a higher maximal retention than white girls, and Chinese-American girls have the lowest maximal retention rates [258–260]. The intake at which the plateau occurs is not different between white and black girls, but it is lower in Chinese-American girls (1300 versus 970 mg/day). White boys have higher peak calcium retention rates than white girls, but the intake at which the plateau occurs is not different [261]. Chinese-American boys had both higher maximal calcium retention rates and intakes for maximal retention (1100 mg/day) than Chinese-American girls [260]. By contrast, Mexican-American boys and girls do not have different rates of calcium retention, and rates are similar to non-Hispanic white boys but are higher than for non-Hispanic white girls [262]. The intake for maximal retention for white adolescents has been established as the RDA for calcium for adolescents [263]. The recommended intakes for Chinese-American, and perhaps other Asian, adolescent girls could be lowered because maximal bone calcium accretion is achieved at lower calcium intakes than whites. However, actual calcium intakes for most Asian adolescent populations are considerably below this lower threshold intake [264]. Few balance studies have been conducted in children other than adolescents.
Numerous studies have been conducted to determine the amount/types of dietary calcium needed for development of maximal bone mass and strength and the ages/stages of development at which calcium intake might be more critical. Furthermore, efforts have been made to elucidate the relationship between calcium intake and physical activity in maximizing skeletal development.
Heaney [265] recently proposed guidelines for systematic reviews of clinical studies of nutrient effects. He proposed that all studies included in a systematic review of nutrient intake should have baseline nutrient status as an entry criterion and should start with subjects at similar baseline nutrient status values. Baseline nutrient status should be suboptimal, especially in the case of a threshold nutrient such as calcium. If subjects are calcium replete, an intervention to increase calcium intake will usually produce a null effect. Heaney also proposed that for inclusion, all studies should use similar doses of the nutrient intervention and that co-nutrient status should be optimized to ensure that the test nutrient is the only nutrient-related factor in the response. However, in reality, few studies meet all of Heaney’s proposed guidelines. The calcium doses of studies found for this review ranged from 500 to 1200 mg/day. Only three studies supplemented with vitamin D, which is an important co-nutrient for bone health. Importantly, all reported baseline calcium intakes were below the Institute of Medicine (IOM)—recommended levels, ranging from 181 to 1199 mg/day. Thus, in studies of children and adolescents whose average calcium intake is deficient, 90 % of the RCTs detected a statistically and biologically significant effect on bone accrual.
In our review of applicable RCTs, we found that designs of calcium supplement studies are inconsistent with regard to baseline calcium intake, supplement dose, optimization of vitamin D, outcome variables (skeletal site, BMD versus BMC versus area), and adjustment for confounders. Nonetheless, we find that calcium supplementation, whether with pills, fortified foods, or dairy, consistently increases gain in skeletal mass and density measures in children and adolescents, usually between 1.0 and 5.0 %. The skeletal sites showing a calcium effect were widely varied among the studies. Some studies did not adjust for body size, which confounds the outcomes because growing children will all have increases in BMC and BMD due to elongation of the skeleton. In addition, there is some evidence that calcium supplementation also increases gain in height [162].
Vitamin D
Prior to the peak bone mass review published in 2000 [1], no vitamin D RCTs in children or adolescents had been published. Eight RCTs have been conducted since then using vitamin D doses ranging from the equivalent of 200–2000 IU/day, and these RCTs have primarily targeted female subjects between the ages of 10 and 17 years [159, 170, 173, 175–178, 180]. Two publications [175, 176] originated from the same Lebanese RCT, the only RCT to include males. Four RCTs conducted in Lebanon, Finland, China, and the United Kingdom provide moderate evidence to support vitamin D supplementation effects on childhood and adolescent bone mineral accrual. Using an intent-to-treat analysis, supplementation was shown to improve hip BMC [175] and geometrical properties of the femoral neck [176] in females, but not males. In subgroup analyses, the vitamin D effect was more pronounced in prepubertal or early pubertal versus postpubertal girls, as well as in those with lower versus higher baseline 25(OH)D. Two RCTs that provided evidence demonstrating improvements in BMC gains after vitamin D supplementation only did so when analyzing results using a compliance-based analysis [177, 178]. Findings by Du et al. [170] and Khadilkar et al. [178] support the beneficial effects of vitamin D supplementation on total body BMC gains; however, unlike the other single-nutrient studies, vitamin D was combined with calcium. The remaining four RCTs [159, 173, 178, 180] did not show significant changes in BMC measures with supplementation, likely due to the use of small sample sizes or low vitamin D intervention doses, in some cases combined with baseline serum 25(OH)D concentrations above a threshold for demonstrating an effect.
Comparing the findings from the RCTs is complicated because of the different methodologies used to assess serum 25(OH)D, including radioimmunoassay (Diasorin), enzyme immunoassay (Immunodiagnostic Systems), and high-performance liquid chromatography. More confidence in the findings is generated because all laboratories participated in the Vitamin D External Quality Assessment Scheme (DEQAS) and met the standards. Moreover, various statistical approaches were employed among RCTs. The RCT that presented the most compelling evidence for a vitamin D effect on bone accrual presented intent-to-treat, unadjusted data [175]. In another study, positive findings were found only when taking into consideration compliance and statistically adjusting for changes in bone area, weight, and maturation [177]. The other RCTs adjusted for one or a combination of other potential confounders, including baseline bone values, bone area, age, maturation, height, weight, fat-free soft tissue, calcium intakes, sunlight exposure, and physical activity.
It is important to note that mean baseline serum 25(OH)D concentrations for all RCTs in our review were between 18 and 48 nmol/L, which are lower than the 50-nmol/L cutoff used to define vitamin D sufficiency [245]. Using changes in BMC as the primary outcome, even in study samples with deficient-to-low serum 25(OH)D, supplementation did not consistently promote gains. In a systematic review by Winzenberg et al. [266], the authors concluded that vitamin D supplementation is more likely to augment hip, forearm and lumbar spine aBMD in children with low serum 25(OH)D concentrations. To our knowledge, no RCTs with bone outcomes have been conducted in children and adolescents with serum 25(OH)D concentrations ≥50 nmol/L [263].
The osteogenic effects of calcitriol are attributed to its role in serum calcium homeostasis, partially through regulation of intestinal calcium absorption [267]. Calcium absorption was not assessed in the four RCTs in this review. One dose–response RCT in children entering the early stages of puberty with a mean baseline serum 25(OH)D of 70 nmol/L showed no effects of supplementation on fractional calcium absorption using vitamin D doses ranging from 400 to 4000 IU over 12 weeks [268]. These results are compatible with the aforementioned systematic review [266], which stated that the beneficial effects of vitamin D on bone may be less likely to occur in children with sufficient serum 25(OH)D.
Although we ranked the evidence for a positive effect of vitamin D supplementation on bone mineral accrual in children and adolescents as moderate, several unanswered questions remain. Only one study was conducted in males, and it is therefore premature to make conclusions regarding sexual dimorphism with respect to vitamin D supplementation and bone. Moreover, subgroup analyses were conducted in several studies in an attempt to identify critical times during childhood and adolescence during which supplementation may be most effective on bone. The results reported for premenarche, the early stages of puberty, or the postmenarche years, however, were inconsistent. These equivocal findings deserve further investigation.
Micronutrients other than calcium and vitamin D
Few trials of any type have been conducted on micronutrients other than calcium and vitamin D relevant to bone health to guide recommendations. The studies on other micronutrients included in this review were not generalizable or were limited in sample size or other aspects of study design. Magnesium, phosphorus, vitamin K, vitamin C, zinc, and other nutrients play important structural and functional roles for bone. Only magnesium was tested in an RCT, but this benefit to BMC was only evaluated in a small group of white girls and only at one level of supplementation. Results of the two studies that found sex differences [189, 190] may be explained by the study group being in a period of active bone modeling. Boys experience peak bone mass accrual later than girls [3]. The benefits of vitamin C in the study by Prynne et al. [189] were not observed in girls of the same age, likely because the girls were more sexually mature. Benefits to younger girls were apparent in the study by Laudermilk et al. [190]. In cross-sectional analyses, inverse relations were observed between phosphorus and sodium intake and total body BMC and size-adjusted bone area [149]. Although phosphorus is an important component of bone mineral, it may be a marker for cola intake (see below under food patterns) or protein intake. The observed negative effect of sodium on bone mass and area may be explained by the negative effect of high dietary sodium intakes on calcium balance through greater urinary calcium excretion demonstrated in adolescent girls [269]. In that study, the negative effect of dietary sodium was more pronounced in white girls than in black girls. Calcium, magnesium, and potassium retention were all greater in black adolescent girls than in white adolescent girls [185, 269–271].
Vitamin K is a cofactor of vitamin K-dependent gamma-carboxylase, an enzyme required for the activation (gamma-carboxylation) of osteocalcin, a protein involved in bone formation and mineralization. Undercarboxylation of osteocalcin, a marker of vitamin K deficiency, was inversely associated with BMC [191]. Kalkwarf et al. [192] were the first to investigate the effects of vitamin K on bone mass and bone turnover in young girls and found little benefit to bone, except in the spine. It should be reemphasized that the girls in this study were aged 3–16 years, representing a broad span in terms of skeletal maturity. Even though experimental data suggest a stimulatory effect of vitamin K on bone formation [272], the relationship between vitamin K nutritional status and development of peak bone mass and strength in humans remains unclear.
Fluoride promotes osteoblast proliferation, increases aBMD in adults, and has been used as a therapeutic option for patients with osteoporosis [273]. The two prospective studies in children and adolescents [186, 274] suggest a possible osteogenic benefit of living in a specific location with higher fluoride concentrations in the water. Two US prospective reports from Iowa [187, 188], however, showed that lifetime fluoride intakes were not associated with BMC in 11- and 15-year-old adolescents. The public health benefits of water fluoridation for dental caries prevention in children are well documented, but the available limited evidence is insufficient to draw conclusions regarding fluoride and bone during growth.
Many of these micronutrients (i.e., calcium, vitamin D, potassium, and for some subgroups, magnesium and vitamins C and A) are shortfall nutrients compared to recommended intakes as determined by the 2015 Dietary Guidelines Advisory Committee [275].
Food patterns
The evidence since 2000 builds on earlier evidence, with additional RCTs showing a benefit to bone owing to the inclusion of dairy products in the diet. Dairy products contain colloidal calcium phosphate protein complexes in the form of casein micelles that have the minerals and nutrients needed for bone growth. Cross-sectional studies show a positive association between fruit and vegetable intake and higher bone mass. The explanation for the benefit of fruit and vegetable intake to bone is not clear. The benefit may be because of the nutrients that they provide, such as potassium, magnesium, and vitamin C [189, 276]; bioactive ingredients from specific fruits and vegetables, such as flavonoids [277]; or their alkaline ash-forming properties [189]. Studies in children on any of these hypotheses are limited. In a 4-year prospective study in German children aged 6–18 years, urinary net acid excretion, a good indicator of total body net endogenous acid load, was unrelated to bone measures [145].
Carbonated beverage and cola consumption was associated with reduced BMC, aBMD, or bone strength and higher fracture in several cross-sectional studies shown in Table 8, especially in girls. The negative effect of cola beverages and caffeine may be directly related to increased urinary calcium with caffeine or to excess phosphorus intake. To explore the potential mechanism by which carbonated beverages result in lower bone accretion and fracture, Kristensen et al. [278] examined biomarkers of bone turnover in a controlled crossover intervention study with 11 men (aged 22–29 years). The authors compared 10 days on a low-calcium diet with cola versus milk added to the diet. The high cola intake was associated with increased bone turnover compared to the period of high milk intake. Alternatively, the negative effect of carbonated beverage consumption on bone may be explained by associated factors including low milk intake, reduced physical activity, and higher BMI [201]. The effect of diet on bone turnover in the study by Kristensen et al. [278] could be due to calcium in milk, rather than the cola, given that dietary calcium reduces bone resorption in adolescent girls [279]. Milk displacement by soft drinks is associated with reduced intakes of calcium and other nutrients found in milk. Regardless of the mechanism, the evidence suggests that cola consumption while on a low-calcium diet can have adverse effects on bone accretion and retention.
Bioactive food components may influence human gut microbial diversity, which in turn may offer a positive impact on skeletal health. The role of the gut microbiome in regulating bone mass was recently demonstrated using a germ-free mouse model [280]. Flavonoids, found ubiquitously in nature in many plant-derived foods, may also have the potential to positively affect bone health. Although our search did not identify any RCTs that assessed the effect of any flavonoid subclass (or polyphenols in general) in children or adolescents, several animal and/or in vitro analyses have shown a biological plausibility for these compounds to affect bone turnover and markers of bone health [277]. Because of their structural similarity to estrogen, soy isoflavones are currently the main class of flavonoids studied for their role in bone health.
Total dietary fiber does not appear to be related to bone accrual, but fibers that are fermentable to short-chain fatty acids in the lower gut by the gut microbiome are associated with increased calcium absorption [281–283]. In the only intervention study of sufficient duration to examine effects on BMC accrual, a combination of short- and long-chained fructooligosaccharides showed a significant benefit [193].
Infant nutrition
Breastfeeding during the first year of life has long been suggested to be optimal for infant nutrition; however, the available scientific literature is conflicting in terms of bone and fracture outcomes. Formula feeding may have the potential to increase short-term BMC and BMD outcomes [215, 216], possibly due to higher amounts of nutrients such as calcium and vitamin D in most infant formulas compared with breast milk (to note, infant formula contents likely vary between and within observational studies). An older landmark RCT [284] supports this hypothesis and reported that during the first 6 months of life, bone accretion is less in infants fed human or low-mineral formula but is greater in the second 6 months of life. Data from observational studies remain inconsistent since 2000. Additional studies addressing the impact of the duration of breastfeeding on peak bone mass development are also needed because current observational data have shown inconsistent results [212–214, 251]. It is important to note potential confounding bias because mothers who breastfeed have been shown to adopt other positive health behaviors for their children that could influence the development of peak bone mass. It is important to note that the American Academy of Pediatrics has recommended that infants who are breast-fed and children and adolescents who consume less than 1 L of vitamin D-fortified milk per day will likely need supplementation to reach 400 IU of vitamin D per day [285].
Data from the RCT assessing the effects of infant formula enriched with palm olein showed positive effects in relation to total body BMC at 3 and 6 months [211]. However, observational studies of children aged 4.5 and 10 years who consumed infant formula with either added palm olein or sn-2 palmitate during their infancy showed no significant differences in total body BMC [220, 221]. Overall, the data suggest that enrichment of infant formula with palm olein may be beneficial during the first 6 months of life.
Adolescent special issues
Detriment of DMPA injections and oral contraceptives
Data are conflicting regarding the effect of combined OCs on bone density among adolescent girls. OC pill use by healthy, white, teenage females did not affect acquisition of peak bone mass in one study [223]. However, studies that have examined the effect of low-dose estrogen OCs suggest otherwise. Some data suggest that long-term treatment with an oral monophasic contraceptive formulation (ethinylestradiol 20 μg + desogestrel 0.150 mg) raises concerns about suboptimal achievement of peak bone mass [286], especially when initiated during the teenage years. The skeletal effects of combined OCs are of greater concern in adolescents compared to their use in adult women [287, 288]. Initiation of combined OCs within the first 3 years after menarche is of particular concern [288]. OCs suppress endogenous estradiol production by suppressing the hypothalamic–pituitary–ovarian axis. There is growing consensus that OCs containing 20 μg of ethinylestradiol interfere with acquisition of peak BMD, although some studies have had inherent limitations including smoking status, small sample size, poor accounting for confounders, and so forth [289].
Contraception via injections of DMPA is associated with skeletal deficit at the spine and hip when used before peak bone mass. DMPA acts on the skeleton mainly through estrogen deficiency [230]. Pharmacological doses of DMPA may also possess selective glucocorticoid activity and can alter the expression of glucocorticoid receptor-regulated genes. However, weight gain on DMPA may mitigate loss of BMD among adolescent users [222]. In addition, bone loss in female adolescents receiving DMPA for contraception is partly or fully reversible following discontinuation of DMPA, with faster recovery at the spine than at the hip [290]. DMPA is still used commonly in adolescents, but with caution given the potential skeletal implications.
Detriment of alcohol
Alcohol abuse is associated with lower aBMD and increased risk of fracture among adults. However, the association between low to moderate alcohol intake and bone density in adults is inconsistent, with low to moderate intakes associated with higher aBMD than that of abstainers in some studies [291]. Likewise, there is little evidence that alcohol intake at levels currently reported in studies among adolescents to date has any effect on attainment of peak bone mass. An important limitation of published studies is the ability to identify the effects of consuming large daily amounts alcohol (>3 servings/day) on bone due to its low reported prevalence in these studies.
There has been large variability among studies in the amount of alcohol consumed by study participants and the classification of alcohol intake from ever tried to number of drinks per day. Overall, the reported alcohol consumption by adolescents studied was relatively low. In some studies, adolescents who consumed alcohol were more likely to smoke [231–235], necessitating statistical adjustment for smoking to investigate the independent effects of alcohol intake on bone.
Binge drinking is an important consideration for adolescents, because about 90 % of the alcohol consumed by adolescents aged <21 years in the USA is in the form of binge drinking [292]. We did not identify any studies that examined the association between binge drinking on bone health in adolescents.
Detriment of smoking
Despite abundant evidence that smoking has many deleterious health effects, cigarette smoking continues to be common among adolescents and adults. In 2011, 18.1 % of high school students in the USA smoked ≥1 cigarettes in the last 30 days and 19.0 % of adults were current smokers [293].
The strength of evidence regarding the association between smoking and bone in adolescence has been limited by methodological challenges in quantifying smoke exposure and the need to disentangle the effects of smoking from other lifestyle factors such as physical activity, dietary calcium intake, and alcohol consumption. Differences in results across studies arise, in part, due to challenges in characterizing exposure and the low prevalence of regular smoking, limiting statistical power. Despite methodological challenges, results of the studies reviewed herein support the contention that smoking in adolescents may reduce peak bone mass. The large studies of young adult military recruits provide additional evidence that a history of smoking has deleterious effects on bone. Even if the effect of smoking during adolescence on bone mass is small, it may become important if the deleterious effects of smoking on aBMD compound over time. Adolescents who smoke often continue smoking in adulthood, possibly increasing their risk of osteoporosis and fracture later in life.
If the associations between active and passive smoke exposure and aBMD are causally related, curtailment of active and passive smoke exposure to children of all ages will likely facilitate maximal attainment of peak bone mass [294].
Physical activity and exercise
We judged the evidence of a positive effect of physical activity on mass and density as strong (Level A). The evidence is less clear in support of a positive effect of physical activity on structure; therefore, we judged the evidence to be moderate at this time (Level B). Similar bone structure RCT designs have resulted in positive effects [28, 81, 250, 295, 296], no effects [25, 244, 297–303], and different effects based on gender or maturity status [73]. However, despite the inconsistencies in RCT results, the evidence provided by well-designed RCTs [81] and prospective cohort studies [252] supports a positive effect on structure, including those using objective measures of physical activity [248]. Unlike RCTs with mass and density outcomes, the multitude of structural measures, sites for measurement (distal, proximal), and inconsistencies in adjustment for bone size present a unique challenge in evaluating the quality of studies examining and interpreting exercise effects on structure. A design limitation in most of the reviewed RCT studies (mass, density, and structure) was an inability to adequately assess the following: the physical activity levels of controls, the degree of effort in the exercisers, and the activity levels of the exercisers during periods of nonintervention. In short, issues of compliance were common threats to internal validity. In addition, physical activity interventions, in general, are susceptible to compensation effects (i.e., the intervention group does less physical activity outside of the intervention session to maintain a “normal” activity routine) [304].
There is a need to more precisely deliver the exercise dose and to understand the levels of physical activity in control and intervention groups. Laboratory-based work indicates an osteogenic effect at or above mechanical loads of 4.2 g-force [305], whereas RCTs suggest an osteogenic effect at or above 3.5 g-force [24, 306]. RCTs also suggest 3 days/week with 100 loads per session and approximately 7 months of intervention are needed to detect change [24, 306, 307]. Due to the overlap in time and frequency in interventions that show change and those that do not, specific recommendations for these exercise dimensions (time and frequency) are equivocal [25, 73, 295, 301, 302]. At present, 100 loads per session and 3 days/week are reasonable time and frequency dimensions based on successful RCTs [24, 73, 306].
Almost all of the physical activity-related RCTs included in our review used jumping as the primary exercise type. This is a sound decision because jumping is the gross motor skill that mechanically loads the clinically important site of the hip via muscle loading during takeoff and via impact loading during landing. Animal and human studies have shown that jumping imposes a greater anabolic stimulus on bone than running or walking [306, 308], and the latter are activities commonly prescribed for metabolic health and obesity prevention. The known differences in types of physical activities for different targeted health outcomes suggest a need to promote physical activities that incorporate multiple motor skills (e.g., soccer, tumbling, tennis) or promote diverse physical activity patterns.
In addition to the RCTs and prospective longitudinal studies that we reviewed and graded, other types of research support physical activity as a causal factor for healthy bone mass, density, and structure [309]. Many of the mechanisms and pathways have been elucidated in laboratory studies [82, 308, 310] and the theoretical underpinning of why physical activity is expected to influence mass, density, and structure is clearly described in Frost’s mechanostat model [77, 78], which is well respected in the greater scientific community [311–313].
Research gaps
We have identified many questions that will drive a future research agenda (Table 15). Trials should be designed to obtain at least B-level evidence. The following areas merit further investigation: differing effects of interventions depending on the life stage of growth; gene–environment interactions and how they may impact the development of peak bone mass; the need to identify and utilize biomarkers of exposure and effects; and the interaction of bone with other tissues throughout the body. It is important to recognize that the pediatric skeleton with open epiphyses differs from that of a fully grown adult who has reached his or her peak bone mass, and therefore, meaningful clinical targets and response to interventions will also differ. In addition, longitudinal studies are needed to document the relationship between growth and measures of bone fragility and fractures and to identify lifestyle interventions that may prevent fractures during this period of susceptibility.
Table 15.
Future research agenda
| Topic area | What we need to know |
|---|---|
| Life stages of growth for interventions | Are interventions more effective during different stages of growth (e.g., rapid or slow)? Can deficiencies in one stage be overcome subsequently? |
| Is there an influence of fetal programming? | |
| What are the most effective diet and physical activity interventions at each stage? | |
| What is the influence of diet and physical activity patterns, in the short-term and over long periods? | |
| What are the determinants of bone acquisition and the impact of interventions in the understudied period of late adolescence to early adulthood? | |
| Does response to intervention vary by factors such as sex and population ancestry? | |
| Are there other understudied or unstudied lifestyle or environmental factors affect peak bone mass development (i.e., sleep, stress, etc.)? | |
| Gene–environment interactions | Are there interactions that affect peak bone mass development? |
| Biomarkers of exposure and effect | How do we generate better markers of nutritional status, physical activity and bone loading, and other environmental exposures? |
| Among adolescents, exposures to consider include lifestyle habits such as smoking (both nicotine and marijuana) and alcohol, among others. | |
| How do we generate better markers of stage of maturity, peak bone strength development, and associated intermediate mechanisms? | |
| Attention to the multiple factors involved in bone and mineral metabolism is needed in interpreting responses to dietary interventions, including a focus on interactions between vitamin D, phosphorus, calcium, and fibroblast growth factor 23. | |
| Organ and tissue interactions | What are bone interactions with other tissues (i.e., brain, fat, muscle, gut, etc.) on development of peak bone mass? |
Statistical guidelines
Analysis and interpretation of data from studies examining the effects of nutrition, dietary components, and physical activity on bone mass and/or strength requires thoughtful consideration (Table 16). Foremost is the baseline of the dietary or physical activity exposure in the study population. This is particularly important for threshold nutrients or dietary components that do not have a linear association with bone measures across a broad range of intakes. Animal studies also indicate that the effects of physical activity are likely to saturate [310]. In randomized trials, it is important to select participants who are likely to benefit from additional intake and/or exercise. For example, if usual intake of calcium exceeds requirements, then it is unlikely that any benefit of the intervention will be detected. The duration of the intervention is critical to consider in the design of intervention trials and should be appropriate for the bone measure under study. Changes in calcium balance may be measureable within 3 weeks, whereas measureable changes in bone mass or strength due to a dietary and/or exercise intervention may not be evident for 6–12 months.
Table 16.
Elements to consider in the analysis and interpretation of studies examining the effects of nutrition on bone mass or density
| Element |
|---|
| Usual intake of nutrient or dietary component, nutritional status |
| Duration of intervention (randomized trials) |
| Age |
| Sex |
| Race |
| Maturational stage |
| Body size |
| Physical activity |
| Health status and medication use |
| Baseline bone values (prospective studies) |
Subject characteristics, including age, sex, race, maturational stage, and skeletal or body size, should also be considered in the design and analysis of studies examining the association between nutrition or physical activity and bone outcomes because they are strongly associated with bone measures during growth. Statistical adjustment for these characteristics can dramatically reduce residual variability in regression models and improve statistical power to identify associations among dietary intake, physical activity, and bone. In addition, statistical adjustment may compensate for imbalances in these variables across ranges (observational studies) or among intervention groups (randomized trials). Several approaches have been used to account for skeletal size, the most common being height or bone area. Several chronic medical conditions (e.g., anorexia nervosa, cystic fibrosis, etc.) [314–318] and medications (e.g., glucocorticoids, anticonvulsants) [72, 319] are known to affect bone accrual during growth, and these should be accounted for in the study design or statistical analyses. Finally, prospective studies, both randomized trials and observation studies, should consider adjustment for baseline bone values and exposures to minimize the statistical phenomenon of regression toward the mean.
Implementation
Dietary intakes and physical activity levels of most US youth during the development of peak bone mass do not support maximal bone mass accretion for genetic potential. This increases risk for fracture both during childhood and later in life. Adherence to the US Department of Agriculture (USDA)/US Department of Health and Human Services (HHS) Dietary Guidelines for Americans and HHS Physical Activity Guidelines for Americans is an important and positive step toward ensuring healthy bone growth and/or maintenance throughout the lifecycle.
Diet
The recommended intakes of food groups and nutrients relevant to bone, their bone-related functions, and intake status are given in Tables 17 and 18. Shortfall food groups include dairy, fruits, and vegetables (Table 17). Consequently, intakes of nutrients provided by these food groups often do not meet national recommendations (Table 18). Dairy products provide most of the calcium and vitamin D in the diet as well as high-quality protein and significant amounts of magnesium, potassium, and other essential nutrients. Yet, approximately 66 % of boys and 83 % of girls during the time of peak height velocity do not meet the recommended intakes of milk [320]. Low intakes of fruits and vegetables can lead to insufficient intakes of vitamins A, C, E, and K and potassium. Potassium has only recently been associated with bone health [276]. Additional research is needed to confirm why higher fruit and vegetable intakes seem to contribute to pediatric bone health among cross-sectional studies. At present, continuing to advocate for children and adolescents to obtain recommended intakes of fruits and vegetables as described by the Dietary Guidelines for Americans has no downside, and may offer a potential benefit toward development of peak bone mass.
Table 17.
Recommended and actual intakes and functions of food sources involved in development of peak bone mass
| Food source | Bone-related function | Recommended servingsa | Percentage of population with usual intakes below recommendations | ||||
|---|---|---|---|---|---|---|---|
| Children | Males | Females | Children | Males | Females | ||
| Dairy (cups)b | Intakes correlated with linear growth, bone mass accrual, reduced fracture | 2–3 years: 2 | 9–13 years: 3 | 9–13 years: 3 | 2–3 years: 41 | 9–13 years: 8 | 9–13 years: 84 |
| 4–8 years: 2.5 | 14–18 years: 3 | 14–18 years: 3 | 4–8 years: 42 | 14–18 years: 68 | 14–18 years: 92 | ||
| 19–30 years: 3 | 19–30 years: 3 | 19–30 years: 80 | 19–30 years: 94 | ||||
| Fruits (cups)c | Provide micronutrients for optimal bone growth, preserve bone and calcium economy through acid–base balance | 2–3 years: 1 | 9–13 years: 1.5 | 9–13 years: 1.5 | 2–3 years: 32 | 9–13 years: 78 | 9–13 years: 81 |
| 4–8 years: 1–1.5 | 14–18 years: 2 | 14–18 years: 1.5 | 4–8 years: 63 | 14–18 years: 87 | 14–18 years: 85 | ||
| 19–30 years: 2 | 19–30 years: 2 | 19–30 years: 89 | 19–30 years: 93 | ||||
| Vegetables (cups) | Provide micronutrients for optimal bone growth, preserve bone and calcium economy through acid–base balance | 2–3 years: 1 | 9–13 years: 2.5 | 9–13 years: 2 | 2–3 years: 80 | 9–13 years: 96 | 9–13 years: 95 |
| 4–8 years: 1.5 | 14–18 years: 3 | 14–18 years: 2.5 | 4–8 years: 92 | 14–18 years: 97 | 14–18 years: 99 | ||
| 19–30 years: 3 | 19–30 years: 2.5 | 19–30 years: 93 | 19–30 years: 94 | ||||
aBased on the 2010 Dietary Guidelines for Americans, which may be accessed via http://www.health.gov/dietaryguidelines/dga2010/dietaryguidelines2010.pdf (modified from: http://www.choosemyplate.gov)
bRecommended servings of dairy are determined by age
cRecommended servings of fruits and vegetables are determined by age, sex, and level of physical activity
Table 18.
Recommended and actual intakes and functions of nutrients involved in development of peak bone mass
| Nutrients | Dietary sources | Bone-related function | RDA/AI | EARa,b | Percentage of population with usual intakes < EARc,d (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Children | Males | Females | Children | Males | Females | ≥2 years | 2–18 years | ≥19 years | |||
| Macronutrients | |||||||||||
| Protein (g/day) | Animal products, plants, legumes | Organic component of bone that also promotes bone mineral accrual | 1–3 years: 13 | 9–13 years: 34 | 9–13 years: 34 | 1–3 years: 0.87 | 9–13 years: 0.76 | 9–13 years: 0.76 | <3 | <3 | <3 |
| 4–8 years: 19 | 14–18 years: 52 | 14–18 years: 46 | 4–8 years: 0.76 | 14–18 years: 0.73 | 14–18 years: 0.71 | ||||||
| 19–30 years: 56 | 19–30 years: 46 | 19–30 years: 0.66 | 19–30 years: 0.66 | ||||||||
| Micronutrients | |||||||||||
| Calcium (mg/day) | Dairy, dark leafy greens | Inorganic component of bone essential for rigidity, strength, and elasticity of bone tissue | 1–3 years: 700 | 9–13 years: 1300 | 9–13 years: 1300 | 1–3 years: 500 | 9–13 years: 1100 | 9–13 years: 1100 | 38 | ~47 | ~36 |
| 4–8 years: 1000 | 14–18 years: 1300 | 14–18 years: 1300 | 4–8 years: 800 | 14–18 years: 1100 | 14–18 years: 1100 | ||||||
| 19–30 years: 1000 | 19–30 years: 1000 | 19–30 years: 800 | 19–30 years: 800 | ||||||||
| Phosphorus (mg/day) | Dairy, meat, processed foods, colas | Inorganic component of bone that also functions as an acid–base buffer | 1–3 years: 460 | 9–13 years: 1250 | 9–13 years: 1250 | 1–3 years: 380 | 9–13 years: 1055 | 9–13 years: 1055 | 5 | ~16 | ~1 |
| 4–8 years: 500 | 14–18 years: 1250 | 14–18 years: 1250 | 4–8 years: 405 | 14–18 years: 1055 | 14–18 years: 1055 | ||||||
| 19–30 years: 700 | 19–30 years: 700 | 19–30 years: 580 | 19–30 years: 580 | ||||||||
| Magnesium (mg/day) | Dairy, dark leafy greens, nuts, whole grains | Regulates structural development of bone (i.e., hydroxyapatite) | 1–3 years: 80 | 9–13 years: 240 | 9–13 years: 240 | 1–3 years: 65 | 9–13 years: 200 | 9–13 years: 200 | 45 | ~35 | ~48 |
| 4–8 years: 130 | 14–18 years: 410 | 14–18 years: 360 | 4–8 years: 110 | 14–18 years: 340 | 14–18 years: 300 | ||||||
| 19–30 years: 400 | 19–30 years: 310 | 19–30 years: 330 | 19–30 years: 255 | ||||||||
| Potassium (g/day) | Dairy, fruit (e.g., oranges), vegetables (e.g., potatoes) | Regulation of acid–base balance affecting bone metabolism | 1–3 years: 3.0 | 9–13 years: 4.5 | 9–13 years: 4.5 | – | – | – | 3 (<AI) | – | – |
| 4–8 years: 3.8 | 14–18 years: 4.7 | 14–18 years: 4.7 | |||||||||
| 19–30 years: 4.7 | 19–30 years: 4.7 | ||||||||||
| Zinc (mg/day) | Animal products, nuts, seeds | Required for collagen synthesis and bone formation | 1–3 years: 3 | 9–13 years: 8 | 9–13 years: 8 | 1–3 years: 2.5 | 9–13 years: 7.0 | 9–13 years: 7.0 | 8 | ~5 | ~8 |
| 4–8 years: 5 | 14–18 years: 11 | 14–18 years: 9 | 4–8 years: 4.0 | 14–18 years: 8.5 | 14–18 years: 7.3 | ||||||
| 19–30 years: 11 | 19–30 years: 8 | 19–30 years: 9.4 | 19–30 years: 6.8 | ||||||||
| Iron (mg/day) | Animal products, fruits, vegetables, fortified grain products | Cofactor required for collagen synthesis and vitamin D activation | 1–3 years: 7 | 9–13 years: 8 | 9–13 years: 8 | 1–3 years: 3.0 | 9–13 years: 5.9 | 9–13 years: 5.7 | 5 | ~2 | ~6 |
| 4–8 years: 10 | 14–18 years: 11 | 14–18 years: 15 | 4–8 years: 4.1 | 14–18 years: 7.7 | 14–18 years: 7.9 | ||||||
| 19–30 years: 8 | 19–30 years: 18 | 19–30 years: 6.0 | 19–30 years: 8.1 | ||||||||
| Manganese (mg/day) | Nuts, legumes, whole grains | Cofactor required for proteoglycan synthesis and bone formation | 1–3 years: 1.2 | 9–13 years: 1.9 | 9–13 years: 1.6 | — | — | — | — | — | — |
| 4–8 years: 1.5 | 14–18 years: 2.2 | 14–18 years: 1.6 | |||||||||
| 19–30 years: 2.3 | 19–30 years: 1.8 | ||||||||||
| Vitamin K (μg/day) | Green vegetables, plant oils, margarine | Cofactor required for carboxylation of osteocalcin and bone formation | 1–3 years: 30 | 9–13 years: 60 | 9–13 years: 60 | – | – | – | 35 (<AI) | – | – |
| 4–8 years: 55 | 14–18 years: 75 | 14–18 years: 75 | |||||||||
| 19–30 years: 120 | 19–30 years: 90 | ||||||||||
| Vitamin C (mg/day) | Citrus fruits, dark leafy greens | Cofactor required for cross-linking of collagen fibers | 1–3 years: 15 | 9–13 years: 45 | 9–13 years: 45 | 1–3 years: 13 | 9–13 years: 39 | 9–13 years: 39 | 25 | ~17 | ~28 |
| 4–8 years: 25 | 14–18 years: 75 | 14–18 years: 65 | 4–8 years: 22 | 14–18 years: 63 | 14–18 years: 56 | ||||||
| 19–30 years: 90 | 19–30 years: 75 | 19–30 years: 75 | 19–30 years: 60 | ||||||||
| Vitamin A (μg/day) | Dairy, darkly colored fruits and leafy vegetables | Implicated in bone formation and resorption | 1–3 years: 300 | 9–13 years: 600 | 9–13 years: 600 | 1–3 years: 210 | 9–13 years: 445 | 9–13 years: 420 | 34 | ~28 | ~38 |
| 4–8 years: 400 | 14–18 years: 900 | 14–18 years: 700 | 4–8 years: 275 | 14–18 years: 630 | 14–18 years: 485 | ||||||
| 19–30 years: 900 | 19–30 years: 700 | 19–30 years: 625 | 19–30 years: 500 | ||||||||
| Vitamin D (IU/day) | Fortified dairy, fatty fish | Regulates calcium homeostasis and bone metabolism | 1–3 years: 600 | 9–13 years: 600 | 9–13 years: 600 | 1–3 years: 400 | 9–13 years: 400 | 9–13 years: 400 | 70 | ~75 | ~69 |
| 4–8 years: 600 | 14–18 years: 600 | 14–18 years: 600 | 4–8 years: 400 | 14–18 years: 400 | 14–18 years: 400 | ||||||
| 19–30 years: 600 | 19–30 years: 600 | 19–30 years: 400 | 19–30 years: 400 | ||||||||
AI adequate intake, EAR estimated average requirement, RDA recommended dietary allowance
aRDA is the average daily dietary intake level that is sufficient to meet the nutrient requirements of nearly all (97–98 %) healthy individuals in a group. It is calculated from the EAR, which is the average daily nutrient intake level estimated to meet the requirements of half of the healthy individuals in a group. If sufficient scientific evidence is not available to establish an EAR, and thus calculate an RDA, an AI is usually developed. EARs have not been established for vitamin K, potassium, manganese, or other nutrients not yet evaluated via the DRI process
bData are from the Dietary Reference Intakes for Calcium, Phosphorous, Magnesium, Vitamin D, and Fluoride (1997); Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline (1998); Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids (2000); Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc (2001); Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (2002/2005); and Dietary Reference Intakes for Calcium and Vitamin D (2011). These reports may be accessed via www.nap.edu (modified from: http://www.iom.edu/Activities/Nutrition/SummaryDRIs/DRI-Tables.aspx)
cData are from Fulgoni [366]
dData are from Fulgoni et al. [321]
Recommended intakes of vitamin D are particularly difficult to achieve without fortified foods or supplements. Enriched and fortified foods provide almost 60 % of dietary vitamin D and 30 % of vitamin A as well as substantial amounts of B vitamins and iron [321]. Fortified foods provide most of the vitamin D in the US diet [263, 275]. Most US milk is fortified with 100 IU of vitamin D per cup [263]. Breakfast cereals often contain added vitamin D, as do some brands of orange juice, yogurt, margarine, and soy beverages. The USA requires infant formula to contain a minimum of 40 IU and a maximum of 100 IU of vitamin D per 100 kcal (21 CFR 107.100). Low-income, overweight/obese, and minority populations of children in the USA have been shown to have lower intakes of both vitamin D and calcium [209].
Very few foods naturally have vitamin D. Fatty fish (e.g., salmon, tuna, and mackerel) and fish liver oils are the best sources, whereas beef liver, cheese, and egg yolks contribute small amounts [263]. Some mushrooms naturally provide vitamin D, and mushrooms and yeast are available with enhanced levels of vitamin D from being exposed to ultraviolet light [322–324] but are scarce on the market.
There is recent growing interest in the possibility that intake of 25(OH)D, the metabolized form of vitamin D that is also present in animal foods such as meat, poultry, and eggs, may be contributing to vitamin D status in humans [325]. Amounts of 25(OH)D in foods currently are not included in the USDA food tables. Studies that have reported discrepancies between estimated vitamin D intakes and serum levels of 25(OH)D are driving the interest in determination of 25(OH)D in foods.
Physical activity
Regular physical activity in youth promotes healthier bones throughout childhood and adolescence. As part of the federally recommended ≥60 min of daily physical activity, children and adolescents should include bone-strengthening physical activity at least 3 days of the week. Bone-strengthening activities are those that are dynamic, moderate to high in load magnitude, short in load duration, odd or nonrepetitive in load direction, and applied quickly [84, 326].
Although complete data are lacking, the IOM estimates that only about one half of youth meet the current HHS Physical Activity Guidelines for Americans’ recommendation for ≥60 min of daily moderate-to-vigorous intensity physical activity. The number of youth meeting this recommendation decreases with age [327] and precipitously declines in early adolescence, a time when bone appears most responsive to physical activity. Throughout childhood and adolescence, girls are less active than boys and are clearly missing opportunities to optimize bone health. The US Centers for Disease Control and Prevention (CDC) reports that as many as one third of youth report no physical activity in the preceding 5 days [328]. Regrettably, participation in bone-strengthening physical activities is not measured in the CDC Youth Risk Behavior Surveillance System. Daily opportunities for incidental physical activity have declined for both children and adolescents as a result of factors such as increased reliance on nonactive transportation, automation of activities for daily living, and greater opportunities for sedentary behavior. Disparities in opportunities for physical activity exist across racial, ethnic, and socioeconomic profiles [327].
Taking action
A multilayered approach must be applied to achieving the recommendations of the Dietary and Physical Activity Guidelines for Americans.
Families
Adults must model and participate in healthy behaviors and engage with family members during mealtime and exercise. Government resources such as MyPlate and the Youth Physical Activity Guidelines Toolkit are informative resources for parents.
Schools
Schools must continue to improve and implement optimal nutrition standards through programs such as the National School Lunch Program and the National School Breakfast Program. Specific strategies for creating an optimal environment for inclusive physical education, extracurricular physical activities, and active classrooms are provided in the USA, most recently by the 2012 Physical Activity Guidelines for American’s Midcourse Report: Strategies to Increase Physical Activity Among Youth [329] and the recent IOM report Educating the Student Body: Taking Physical Activity and Physical Education to School [327]. Early knowledge of nutrition and physical activity through consumer sciences and physical education courses should be mandatory in every K–12 school. Recess should be mandatory for every K–5 school.
Healthcare system
All allied healthcare providers should be required to be proficient in both counseling for nutrition and physical activity through their required curriculum and continuing education. Scientific groups such as the National Osteoporosis Foundation, the American Society for Nutrition, the Academy of Nutrition and Dietetics, the American College of Sports Medicine, and the Society for Health and Physical Educators among others should provide tool kits and educational materials with consistent messaging on nutrition and physical activity for bone health to allied healthcare providers.
Federal, state, and local policy
Government support for healthy growth and development should reach beyond obesity to antecedents of chronic disease, including osteoporosis. Subsidizing foods for bone health through programs such as Head Start, the National School Breakfast Program, the National School Lunch Program, the Supplemental Nutrition Assistance Program, and the Special Supplemental Nutrition Program for Women, Infants, and Children helps to ensure that all children meet their nutrition requirements. Examples of how physical activity requirements are supported include the Federal Safe Routes to Schools Program and the Let’s Move program, as well as public–private sector collaborations such as the NFL Play 60 Challenge and the Partnership for a Healthier America. Through zoning, incentives, and innovative cooperative agreements with businesses, local governments can help to ensure access to fresh food as well as parks and youth recreation opportunities. Expanding successful federal, state, and local nutrition and physical activity programs as well as facilitating innovative collaboration between the public and private sectors are critical to creating a society in which bone health matters.
Conclusions
There is a critical need for more research focusing on bone health in youth. Future research should consider sex, population ancestry, and maturation. When possible, standardizing outcome measures would facilitate the pooling of data for evidence-based reviews.
The best evidence is available for positive effects of calcium intake and physical activity, especially during the late childhood and peripubertal years—a critical period for bone accretion. Good evidence is also available for a role of vitamin D and dairy consumption. However, more work is needed on physical activity dose response and the potential interaction between physical activity and diet quality. Weaker but physiologically plausible evidence is available and emerging for the effects of macronutrients and other micronutrients on bone among youth. It is important to address the factors most strongly linked to developing peak bone mass and strength from the current evidence through multilayered public health strategies. It is equally important to develop a research agenda to better understand other lifestyle factors that are less clearly understood for the purpose of building strong and healthy bones. Meanwhile, meeting federal guidelines for intakes of nutrients and physical activity while cautioning against harmful behaviors is a priority strategy.
Glossary
| Terminology | Acronym | Definition |
| Areal bone mineral density | aBMD | DXA calculates BMD using area. This is not an accurate measurement of the true bone mineral density, which is mass divided by volume. It is a reasonable estimate of BMC. |
| Bone mineral content | BMC | DXA measures the BMC of the spine, hip, wrist, femur, or any other selected part of the skeleton. It does this by focusing an x-ray on a body site and measuring the proportion of light rays that pass through the tissue as opposed to being blocked by minerals in the bone. Using computer software, it then divides that number by the surface area of the bone being measured to create BMD. |
| Bone mineral density | BMD | BMD refers to the amount of mineral matter per square centimeter of bone. BMD is used as a predictor of osteoporosis and fracture risk. |
| Computed tomography | CT | CT is an imaging procedure that uses special x-ray equipment to create a series of detailed pictures, or scans, of areas inside the body. It is also called computerized tomography and computerized axial tomography (CAT) scanning. |
| Cross-sectional moment of inertia | CSMI | CSMI is a measure of the distribution of material around a given axis. It is used to calculate bending stress. |
| Dual-energy x-ray absorptiometry | DXA | DXA is a means of measuring BMD. It is the most widely used and most thoroughly studied bone density measurement technology. Two x-ray beams with different energy levels are aimed at the patient’s bones. When soft tissue absorption is subtracted out, the BMD can be determined from the absorption of each beam by bone. |
| Hip structural analysis | HSA | HSA measures not only the BMD of the hip bone but also structural geometry of cross-sections traversing the proximal femur at specific locations. The bone mass image is used directly from the DXA scan, where pixel values are expressed in areal mass (g/cm2). The method employs the principle that a line of pixel values across the bone axis corresponds to a cut plane traversing the bone at that location and contains some of the information about the cross-section. |
| Percentage of undercarboxylated osteocalcin | %ucOC | %ucOC is a measure of vitamin K status. Osteocalcin is a vitamin K-dependent protein produced by the bone. The ratio of undercarboxylated to carboxylated or total osteocalcin has been regarded as a marker of inadequate vitamin K status. |
| Peripheral quantitative computed tomography | pQCT | pQCT is a type of quantitative CT used for making measurements of the BMD in a peripheral part of the body, such as the forearms or legs, as opposed to CT that measures BMD at the hip and spine. pQCT is useful for measuring bone strength. |
| Potential renal acid load | PRAL | PRAL is a measure of the acidic or basic effects that a food has on the body. |
| Quantitative computed tomography | QCT | QCT measures BMD using a standard CT scanner with a calibration standard to convert Hounsfield units (HU) of the CT image to BMD values. QCT scans are primarily used to evaluate BMD at the lumbar spine and hip. |
| Stress–strain index | SSI | The SSI of a bone is a surrogate measure of bone strength determined from a cross-sectional scan by QCT or pQCT. The SSI is used to compare the structural parameters determined by analysis of QCT/pQCT cross-sectional scans to the results of a three-point bending test. |
| Volumetric bone mineral density | vBMD | In addition to aBMD using DXA, a projected posteroanterior lateral vertebral scan is added to measure vertebral width, height, and depth to estimate vBMD. This permits direct measurement of bone depth, rather than estimation of projected posteroanterior dimensions. |
Abbreviations
- %ucOC
Percentage of undercarboxylated osteocalcin
- 95 % CI
95 % Confidence interval
- aBMD
Areal bone mineral density
- BMC
Bone mineral content
- CDC
US Centers for Disease Control and Prevention
- CSA
Cross-sectional area
- CSMI
Cross-sectional moment of inertia
- CT
Computed tomography
- DEQAS
Vitamin D External Quality Assessment Scheme
- DMPA
Depot medroxyprogesterone acetate
- DONALD
Dortmund Nutritional and Anthropometric Longitudinally Designed
- DXA
Dual-energy x-ray absorptiometry
- HHS
US Department of Health and Human Services
- HRpQCT
High-resolution peripheral quantitative computed tomography
- HSA
Hip structural analysis
- IGF
Insulin-like growth factor
- IOM
Institute of Medicine
- NHANES
National Health and Nutrition Examination Survey
- OC
Oral contraceptive
- OR
Odds ratio
- pQCT
Peripheral quantitative computed tomography
- PRAL
Potential renal acid load
- QCT
Quantitative computed tomography
- RCT
Randomized controlled trial
- RDA
Recommended dietary allowance
- SSI
Stress–strain index
- UHT
Ultra-heat-treated
- uN
Urinary nitrogen
- USDA
US Department of Agriculture
- vBMD
Volumetric bone mineral density
Compliance with ethical standards
Endorsing societies
This scientific statement has been reviewed and endorsed by the following scientific societies:
American Bone Health
American College of Sports Medicine
Endocrine Society
National Osteoporosis Foundation
Society for Women’s Health Research
Reviewers
This scientific statement was reviewed in draft form by individuals chosen for their diverse perspectives and technical expertise. The purpose of this independent review is to provide candid and critical comments that will assist the National Osteoporosis Foundation in making this published report as sound as possible and to ensure that the report meets standards for both objectivity and evidence. We thank the following individuals for their peer review of this scientific statement:
Sue A. Shapses, PhD
Rutgers, The State University of New Jersey
Kimberly O’Brien, PhD
Cornell University
Urszula T. Iwaniec, PhD
Oregon State University
This scientific statement was peer reviewed by the National Osteoporosis Foundation Research Committee and Osteoporosis International. This scientific statement was approved by the National Osteoporosis Foundation Board of Directors.
Sources of financial support
Funding for the manuscript was provided by the Alliance for Potato Research and the Dairy Research Institute.
Conflicts of interest
TCW is employed by the National Osteoporosis Foundation. CMW, CMG, KFJ, HJK, JML, RL, MO, and BSZ have no disclosures.
Footnotes
Author names for PubMed indexing
Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, O’Karma M, Wallace TC, Zemel BS
An erratum to this article is available online at https://doi.org/10.1007/s00198-016-3551-5.
Change history
3/2/2016
An erratum to this article has been published.
References
- 1.Heaney RP, Abrams S, Dawson-Hughes B, Looker A, Marcus R, Matkovic V, Weaver C. Peak bone mass. Osteoporos Int. 2000;11:985–1009. doi: 10.1007/s001980070020. [DOI] [PubMed] [Google Scholar]
- 2.Parfitt AM. The two faces of growth: benefits and risks to bone integrity. Osteoporos Int. 1994;4:382–398. doi: 10.1007/BF01622201. [DOI] [PubMed] [Google Scholar]
- 3.Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the University of Saskatchewan Bone Mineral Accrual Study. J Bone Miner Res. 1999;14:1672–1679. doi: 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- 4.Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res. 2011;26:1729–1739. doi: 10.1002/jbmr.412. [DOI] [PubMed] [Google Scholar]
- 5.Gilsanz V, Roe TF, Mora S, Costin G, Goodman WG. Changes in vertebral bone density in black girls and white girls during childhood and puberty. N Engl J Med. 1991;325:1597–1600. doi: 10.1056/NEJM199112053252302. [DOI] [PubMed] [Google Scholar]
- 6.Gilsanz V, Skaggs DL, Kovanlikaya A, Sayre J, Loro ML, Kaufman F, Korenman SG. Differential effect of race on the axial and appendicular skeletons of children. J Clin Endocrinol Metab. 1998;83:1420–1427. doi: 10.1210/jcem.83.5.4765. [DOI] [PubMed] [Google Scholar]
- 7.Kirmani S, Christen D, van Lenthe GH, Fischer PR, Bouxsein ML, McCready LK, Melton LJ, 3rd, Riggs BL, Amin S, Muller R, Khosla S. Bone structure at the distal radius during adolescent growth. J Bone Miner Res. 2009;24:1033–1042. doi: 10.1359/JBMR.081255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Q, Wang XF, Iuliano-Burns S, Ghasem-Zadeh A, Zebaze R, Seeman E. Rapid growth produces transient cortical weakness: a risk factor for metaphyseal fractures during puberty. J Bone Miner Res. 2010;25:1521–1526. doi: 10.1002/jbmr.46. [DOI] [PubMed] [Google Scholar]
- 9.Riggs BL, Melton LJ, Robb RA, Camp JJ, Atkinson EJ, McDaniel L, Amin S, Rouleau PA, Khosla S. A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res. 2008;23:205–214. doi: 10.1359/JBMR.071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger C, Goltzman D, Langsetmo L, Joseph L, Jackson S, Kreiger N, Tenenhouse A, Davison KS, Josse RG, Prior JC, Hanley DA. Peak bone mass from longitudinal data: implications for the prevalence, pathophysiology, and diagnosis of osteoporosis. J Bone Miner Res. 2010;25:1948–1957. doi: 10.1002/jbmr.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalkwarf HJ, Laor T, Bean JA. Fracture risk in children with a forearm injury is associated with volumetric bone density and cortical area (by peripheral QCT) and areal bone density (by DXA) Osteoporos Int. 2011;22:607–616. doi: 10.1007/s00198-010-1333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report Series 843. Geneva: World Health Organization; 1994. [PubMed] [Google Scholar]
- 13.Holloway KL, Brennan SL, Kotowicz MA, Bucki-Smith G, Timney EN, Dobbins AG, Williams LJ, Pasco JA. Prior fracture as a risk factor for future fracture in an Australian cohort. Osteoporos Int. 2015;26:629–635. doi: 10.1007/s00198-014-2897-9. [DOI] [PubMed] [Google Scholar]
- 14.Cooper C, Dennison EM, Leufkens HG, Bishop N, van Staa TP. Epidemiology of childhood fractures in Britain: a study using the general practice research database. J Bone Miner Res. 2004;19:1976–1981. doi: 10.1359/JBMR.040902. [DOI] [PubMed] [Google Scholar]
- 15.Landin LA. Fracture patterns in children. Analysis of 8,682 fractures with special reference to incidence, etiology and secular changes in a Swedish urban population 1950–1979. Acta Orthop Scand Suppl. 1983;202:1–109. [PubMed] [Google Scholar]
- 16.Mayranpaa MK, Makitie O, Kallio PE. Decreasing incidence and changing pattern of childhood fractures: a population-based study. J Bone Miner Res. 2010;25:2752–2759. doi: 10.1002/jbmr.155. [DOI] [PubMed] [Google Scholar]
- 17.Yeh FJ, Grant AM, Williams SM, Goulding A. Children who experience their first fracture at a young age have high rates of fracture. Osteoporos Int. 2006;17:267–272. doi: 10.1007/s00198-005-2009-y. [DOI] [PubMed] [Google Scholar]
- 18.de Putter CE, van Beeck EF, Looman CW, Toet H, Hovius SE, Selles RW. Trends in wrist fractures in children and adolescents, 1997–2009. J Hand Surg [Am] 2011;36:1810–1815.e1812. doi: 10.1016/j.jhsa.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Jones G, Boon P. Which bone mass measures discriminate adolescents who have fractured from those who have not? Osteoporos Int. 2008;19:251–255. doi: 10.1007/s00198-007-0458-1. [DOI] [PubMed] [Google Scholar]
- 20.Lyons RA, Delahunty AM, Kraus D, Heaven M, McCabe M, Allen H, Nash P. Children’s fractures: a population based study. Inj Prev. 1999;5:129–132. doi: 10.1136/ip.5.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark EM, Ness AR, Tobias JH. Vigorous physical activity increases fracture risk in children irrespective of bone mass: a prospective study of the independent risk factors for fractures in healthy children. J Bone Miner Res. 2008;23:1012–1022. doi: 10.1359/jbmr.080303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baxter-Jones AD, Kontulainen SA, Faulkner RA, Bailey DA. A longitudinal study of the relationship of physical activity to bone mineral accrual from adolescence to young adulthood. Bone. 2008;43:1101–1107. doi: 10.1016/j.bone.2008.07.245. [DOI] [PubMed] [Google Scholar]
- 23.Detter FT, Rosengren BE, Dencker M, Nilsson JA, Karlsson MK. A 5-year exercise program in pre- and peripubertal children improves bone mass and bone size without affecting fracture risk. Calcif Tissue Int. 2013;92:385–393. doi: 10.1007/s00223-012-9691-5. [DOI] [PubMed] [Google Scholar]
- 24.Gunter K, Baxter-Jones AD, Mirwald RL, Almstedt H, Fuchs RK, Durski S, Snow C. Impact exercise increases BMC during growth: an 8-year longitudinal study. J Bone Miner Res. 2008;23:986–993. doi: 10.1359/JBMR.071201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macdonald HM, Kontulainen SA, Khan KM, McKay HA. Is a school-based physical activity intervention effective for increasing tibial bone strength in boys and girls? J Bone Miner Res. 2007;22:434–446. doi: 10.1359/jbmr.061205. [DOI] [PubMed] [Google Scholar]
- 26.Mackelvie KJ, McKay HA, Khan KM, Crocker PR. A school-based exercise intervention augments bone mineral accrual in early pubertal girls. J Pediatr. 2001;139:501–508. doi: 10.1067/mpd.2001.118190. [DOI] [PubMed] [Google Scholar]
- 27.Slemenda CW, Miller JZ, Hui SL, Reister TK, Johnston CCJ. Role of physical activity in the development of skeletal mass in children. J Bone Miner Res. 1991;6:1227–1233. doi: 10.1002/jbmr.5650061113. [DOI] [PubMed] [Google Scholar]
- 28.Lofgren B, Dencker M, Nilsson JA, Karlsson MK. A 4-year exercise program in children increases bone mass without increasing fracture risk. Pediatrics. 2012;129:e1468–e1476. doi: 10.1542/peds.2011-2274. [DOI] [PubMed] [Google Scholar]
- 29.Khosla S, Melton LJ, 3rd, Dekutoski MB, Achenbach SJ, Oberg AL, Riggs BL. Incidence of childhood distal forearm fractures over 30 years: a population-based study. JAMA. 2003;290:1479–1485. doi: 10.1001/jama.290.11.1479. [DOI] [PubMed] [Google Scholar]
- 30.Kelsey JL, Browner WS, Seeley DG, Nevitt MC, Cummings SR. Risk factors for fractures of the distal forearm and proximal humerus. The Study of Osteoporotic Fractures Research Group. Am J Epidemiol. 1992;135:477–489. doi: 10.1093/oxfordjournals.aje.a116314. [DOI] [PubMed] [Google Scholar]
- 31.Clark EM, Ness AR, Bishop NJ, Tobias JH. Association between bone mass and fractures in children: a prospective cohort study. J Bone Miner Res. 2006;21:1489–1495. doi: 10.1359/jbmr.060601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, Hangartner TN, Huang X, Frederick MM, Winer KK, Zemel BS. Tracking of bone mass and density during childhood and adolescence. J Clin Endocrinol Metab. 2010;95:1690–1698. doi: 10.1210/jc.2009-2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowden LS, Jones CJ, Ryan SW. Bone mineralisation in ex-preterm infants aged 8 years. Eur J Pediatr. 1999;158:658–661. doi: 10.1007/s004310051171. [DOI] [PubMed] [Google Scholar]
- 34.Dennison EM, Syddall HE, Sayer AA, Gilbody HJ, Cooper C. Birth weight and weight at 1 year are independent determinants of bone mass in the seventh decade: the Hertfordshire cohort study. Pediatr Res. 2005;57:582–586. doi: 10.1203/01.PDR.0000155754.67821.CA. [DOI] [PubMed] [Google Scholar]
- 35.Farr JN, Amin S, Melton LJ, 3rd, Kirmani S, McCready LK, Atkinson EJ, Muller R, Khosla S. Bone strength and structural deficits in children and adolescents with a distal forearm fracture resulting from mild trauma. J Bone Miner Res. 2014;29:590–599. doi: 10.1002/jbmr.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thandrayen K, Norris SA, Pettifor JM. Fracture rates in urban South African children of different ethnic origins: the Birth to Twenty cohort. Osteoporos Int. 2009;20:47–52. doi: 10.1007/s00198-008-0627-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wren TA, Shepherd JA, Kalkwarf HJ, Zemel BS, Lappe JM, Oberfield S, Dorey FJ, Winer KK, Gilsanz V. Racial disparity in fracture risk between white and nonwhite children in the United States. J Pediatr. 2012;161:1035–1040. doi: 10.1016/j.jpeds.2012.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cauley JA, Lui LY, Ensrud KE, Zmuda JM, Stone KL, Hochberg MC, Cummings SR. Bone mineral density and the risk of incident nonspinal fractures in black and white women. JAMA. 2005;293:2102–2108. doi: 10.1001/jama.293.17.2102. [DOI] [PubMed] [Google Scholar]
- 39.Shin MH, Zmuda JM, Barrett-Connor E, Sheu Y, Patrick AL, Leung PC, Kwok A, Kweon SS, Nam HS, Cauley JA. Race/ethnic differences in associations between bone mineral density and fracture history in older men. Osteoporos Int. 2014;25:837–845. doi: 10.1007/s00198-013-2503-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loud KJ, Gordon CM, Micheli LJ, Field AE. Correlates of stress fractures among preadolescent and adolescent girls. Pediatrics. 2005;115:e399–e406. doi: 10.1542/peds.2004-1868. [DOI] [PubMed] [Google Scholar]
- 41.Hame SL, LaFemina JM, McAllister DR, Schaadt GW, Dorey FJ. Fractures in the collegiate athlete. Am J Sports Med. 2004;32:446–451. doi: 10.1177/0363546503261708. [DOI] [PubMed] [Google Scholar]
- 42.Lappe JM, Stegman MR, Recker RR. The impact of lifestyle factors on stress fractures in female army recruits. Osteoporos Int. 2001;12:35–42. doi: 10.1007/s001980170155. [DOI] [PubMed] [Google Scholar]
- 43.Mattila VM, Niva M, Kiuru M, Pihlajamaki H. Risk factors for bone stress injuries: a follow-up study of 102,515 person-years. Med Sci Sports Exerc. 2007;39:1061–1066. doi: 10.1249/01.mss.0b013e318053721d. [DOI] [PubMed] [Google Scholar]
- 44.Cosman F, Ruffing J, Zion M, Uhorchak J, Ralston S, Tendy S, McGuigan FE, Lindsay R, Nieves J. Determinants of stress fracture risk in United States Military Academy cadets. Bone. 2013;55:359–366. doi: 10.1016/j.bone.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Wentz L, Liu PY, Haymes E, Ilich JZ. Females have a greater incidence of stress fractures than males in both military and athletic populations: a systemic review. Mil Med. 2011;176:420–430. doi: 10.7205/milmed-d-10-00322. [DOI] [PubMed] [Google Scholar]
- 46.Knapik J, Montain SJ, McGraw S, Grier T, Ely M, Jones BH. Stress fracture risk factors in basic combat training. Int J Sports Med. 2012;33:940–946. doi: 10.1055/s-0032-1311583. [DOI] [PubMed] [Google Scholar]
- 47.Friedl KE, Evans RK, Moran DS. Stress fracture and military medical readiness: bridging basic and applied research. Med Sci Sports Exerc. 2008;40:S609–S622. doi: 10.1249/MSS.0b013e3181892d53. [DOI] [PubMed] [Google Scholar]
- 48.Gam A, Goldstein L, Karmon Y, Mintser I, Grotto I, Guri A, Goldberg A, Ohana N, Onn E, Levi Y, Bar-Dayan Y. Comparison of stress fractures of male and female recruits during basic training in the Israeli anti-aircraft forces. Mil Med. 2005;170:710–712. doi: 10.7205/milmed.170.8.710. [DOI] [PubMed] [Google Scholar]
- 49.Lappe J, Davies K, Recker R, Heaney R. Quantitative ultrasound: use in screening for susceptibility to stress fractures in female army recruits. J Bone Miner Res. 2005;20:571–578. doi: 10.1359/JBMR.041208. [DOI] [PubMed] [Google Scholar]
- 50.Field AE, Gordon CM, Pierce LM, Ramappa A, Kocher MS. Prospective study of physical activity and risk of developing a stress fracture among preadolescent and adolescent girls. Arch Pediatr Adolesc Med. 2011;165:723–728. doi: 10.1001/archpediatrics.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loud KJ, Micheli LJ, Bristol S, Austin SB, Gordon CM. Family history predicts stress fracture in active female adolescents. Pediatrics. 2007;120:e364–e372. doi: 10.1542/peds.2006-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friedl KE, Nuovo JA, Patience TH, Dettori JR. Factors associated with stress fracture in young army women: indications for further research. Mil Med. 1992;157:334–338. [PubMed] [Google Scholar]
- 53.Budek AZ, Mark T, Michaelsen KF, Molgaard C. Tracking of size-adjusted bone mineral content and bone area in boys and girls from 10 to 17 years of age. Osteoporos Int. 2010;21:179–182. doi: 10.1007/s00198-009-0932-z. [DOI] [PubMed] [Google Scholar]
- 54.Cheng S, Volgyi E, Tylavsky FA, Lyytikainen A, Tormakangas T, Xu L, Cheng SM, Kroger H, Alen M, Kujala UM. Trait-specific tracking and determinants of body composition: a 7-year follow-up study of pubertal growth in girls. BMC Med. 2009;7:5. doi: 10.1186/1741-7015-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Foley S, Quinn S, Jones G. Tracking of bone mass from childhood to adolescence and factors that predict deviation from tracking. Bone. 2009;44:752–757. doi: 10.1016/j.bone.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 56.Fujita Y, Iki M, Ikeda Y, Morita A, Matsukura T, Nishino H, Yamagami T, Kagamimori S, Kagawa Y, Yoneshima H. Tracking of appendicular bone mineral density for 6 years including the pubertal growth spurt: Japanese population-based osteoporosis kids cohort study. J Bone Miner Metab. 2011;29:208–216. doi: 10.1007/s00774-010-0213-0. [DOI] [PubMed] [Google Scholar]
- 57.Wren TA, Kalkwarf HJ, Zemel BS, Lappe JM, Oberfield S, Shepherd JA, Winer KK, Gilsanz V. Longitudinal tracking of dual-energy X-ray absorptiometry bone measures over 6 years in children and adolescents: persistence of low bone mass to maturity. J Pediatr. 2014;164:1280–1285.e1282. doi: 10.1016/j.jpeds.2013.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lorentzon M, Mellstrom D, Ohlsson C. Age of attainment of peak bone mass is site specific in Swedish men—the GOOD study. J Bone Miner Res. 2005;20:1223–1227. doi: 10.1359/JBMR.050306. [DOI] [PubMed] [Google Scholar]
- 59.Thomas SR, Kalkwarf HJ, Buckley DD, Heubi JE. Effective dose of dual-energy X-ray absorptiometry scans in children as a function of age. J Clin Densitom. 2005;8:415–422. doi: 10.1385/jcd:8:4:415. [DOI] [PubMed] [Google Scholar]
- 60.Crabtree NJ, Arabi A, Bachrach LK, Fewtrell M, El-Hajj Fuleihan G, Kecskemethy HH, Jaworski M, Gordon CM. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J Clin Densitom. 2014;17:225–242. doi: 10.1016/j.jocd.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Zemel BS, Leonard MB, Kelly A, Lappe JM, Gilsanz V, Oberfield S, Mahboubi S, Shepherd JA, Hangartner TN, Frederick MM, Winer KK, Kalkwarf HJ. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95:1265–1273. doi: 10.1210/jc.2009-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wren TA, Liu X, Pitukcheewanont P, Gilsanz V. Bone acquisition in healthy children and adolescents: comparisons of dual-energy x-ray absorptiometry and computed tomography measures. J Clin Endocrinol Metab. 2005;90:1925–1928. doi: 10.1210/jc.2004-1351. [DOI] [PubMed] [Google Scholar]
- 63.Prentice A, Parsons TJ, Cole TJ. Uncritical use of bone mineral density in absorptiometry may lead to size-related artifacts in the identification of bone mineral determinants. Am J Clin Nutr. 1994;60:837–842. doi: 10.1093/ajcn/60.6.837. [DOI] [PubMed] [Google Scholar]
- 64.Molgaard C, Thomsen BL, Prentice A, Cole TJ, Michaelsen KF. Whole body bone mineral content in healthy children and adolescents. Arch Dis Child. 1997;76:9–15. doi: 10.1136/adc.76.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hogler W, Briody J, Woodhead HJ, Chan A, Cowell CT. Importance of lean mass in the interpretation of total body densitometry in children and adolescents. J Pediatr. 2003;143:81–88. doi: 10.1016/S0022-3476(03)00187-2. [DOI] [PubMed] [Google Scholar]
- 66.Crabtree NJ, Kibirige MS, Fordham JN, Banks LM, Muntoni F, Chinn D, Boivin CM, Shaw NJ. The relationship between lean body mass and bone mineral content in paediatric health and disease. Bone. 2004;35:965–972. doi: 10.1016/j.bone.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 67.Short DF, Zemel BS, Gilsanz V, Kalkwarf HJ, Lappe JM, Mahboubi S, Oberfield SE, Shepherd JA, Winer KK, Hangartner TN. Fitting of bone mineral density with consideration of anthropometric parameters. Osteoporos Int. 2011;22:1047–1057. doi: 10.1007/s00198-010-1284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Horlick M, Wang J, Pierson RNJ, Thornton JC. Prediction models for evaluation of total-body bone mass with dual-energy X-ray absorptiometry among children and adolescents. Pediatrics. 2004;114:e33–e45. doi: 10.1542/peds.2004-0301. [DOI] [PubMed] [Google Scholar]
- 69.Crabtree NJ, Hogler W, Cooper MS, Shaw NJ. Diagnostic evaluation of bone densitometric size adjustment techniques in children with and without low trauma fractures. Osteoporos Int. 2013;24:2015–2024. doi: 10.1007/s00198-012-2263-8. [DOI] [PubMed] [Google Scholar]
- 70.Beck TJ, Ruff CB, Warden KE, Scott WWJ, Rao GU. Predicting femoral neck strength from bone mineral data. A structural approach. Invest Radiol. 1990;25:6–18. doi: 10.1097/00004424-199001000-00004. [DOI] [PubMed] [Google Scholar]
- 71.Beck TJ, Stone KL, Oreskovic TL, Hochberg MC, Nevitt MC, Genant HK, Cummings SR. Effects of current and discontinued estrogen replacement therapy on hip structural geometry: the study of osteoporotic fractures. J Bone Miner Res. 2001;16:2103–2110. doi: 10.1359/jbmr.2001.16.11.2103. [DOI] [PubMed] [Google Scholar]
- 72.Burnham JM, Shults J, Petit MA, Semeao E, Beck TJ, Zemel BS, Leonard MB. Alterations in proximal femur geometry in children treated with glucocorticoids for Crohn disease or nephrotic syndrome: impact of the underlying disease. J Bone Miner Res. 2007;22:551–559. doi: 10.1359/jbmr.070110. [DOI] [PubMed] [Google Scholar]
- 73.Petit MA, McKay HA, MacKelvie KJ, Heinonen A, Khan KM, Beck TJ. A randomized school-based jumping intervention confers site and maturity-specific benefits on bone structural properties in girls: a hip structural analysis study. J Bone Miner Res. 2002;17:363–372. doi: 10.1359/jbmr.2002.17.3.363. [DOI] [PubMed] [Google Scholar]
- 74.Adams JE, Engelke K, Zemel BS, Ward KA. Quantitative computer tomography in children and adolescents: the 2013 ISCD Pediatric Official Positions. J Clin Densitom. 2014;17:258–274. doi: 10.1016/j.jocd.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 75.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- 76.Bouchard C, Blair SN, Haskell W, editors. Physical activity and health. Champaign: Human Kinetics; 2006. [Google Scholar]
- 77.Frost HM, Schonau E. The “muscle-bone unit” in children and adolescents: a 2000 overview. J Pediatr Endocrinol Metab. 2000;13:571–590. doi: 10.1515/jpem.2000.13.6.571. [DOI] [PubMed] [Google Scholar]
- 78.Frost HM. Bone’s mechanostat: a 2003 update. Anat Rec A: Discov Mol Cell Evol Biol. 2003;275:1081–1101. doi: 10.1002/ar.a.10119. [DOI] [PubMed] [Google Scholar]
- 79.Kannus P, Haapasalo H, Sankelo M, Sievanen H, Pasanen M, Heinonen A, Oja P, Vuori I. Effect of starting age of physical activity on bone mass in the dominant arm of tennis and squash players. Ann Intern Med. 1995;123:27–31. doi: 10.7326/0003-4819-123-1-199507010-00003. [DOI] [PubMed] [Google Scholar]
- 80.Welten DC, Kemper HC, Post GB, Van Mechelen W, Twisk J, Lips P, Teule GJ. Weight-bearing activity during youth is a more important factor for peak bone mass than calcium intake. J Bone Miner Res. 1994;9:1089–1096. doi: 10.1002/jbmr.5650090717. [DOI] [PubMed] [Google Scholar]
- 81.Specker B, Binkley T. Randomized trial of physical activity and calcium supplementation on bone mineral content in 3- to 5-year-old children. J Bone Miner Res. 2003;18:885–892. doi: 10.1359/jbmr.2003.18.5.885. [DOI] [PubMed] [Google Scholar]
- 82.Turner CH, Forwood MR, Rho JY, Yoshikawa T. Mechanical loading thresholds for lamellar and woven bone formation. J Bone Miner Res. 1994;9:87–97. doi: 10.1002/jbmr.5650090113. [DOI] [PubMed] [Google Scholar]
- 83.Forwood MR, Turner CH. Skeletal adaptations to mechanical usage: results from tibial loading studies in rats. Bone. 1995;17:197S–205S. doi: 10.1016/8756-3282(95)00292-l. [DOI] [PubMed] [Google Scholar]
- 84.Turner CH, Robling AG. Designing exercise regimens to increase bone strength. Exerc Sport Sci Rev. 2003;31:45–50. doi: 10.1097/00003677-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 85.Baptista F, Janz K. Physical activity, bone growth, and development in children and adolescents: a public health perspective. In: Preedy VR, editor. Handbook of growth and growth monitoring in health and disease. New York: Springer; 2012. pp. 2395–2411. [Google Scholar]
- 86.Khan K, McKay HA, Haapasalo H, Bennell KL, Forwood MR, Kannus P, Wark JD. Does childhood and adolescence provide a unique opportunity for exercise to strengthen the skeleton? J Sci Med Sport. 2000;3:150–164. doi: 10.1016/s1440-2440(00)80077-8. [DOI] [PubMed] [Google Scholar]
- 87.Duncan RL, Turner CH. Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int. 1995;57:344–358. doi: 10.1007/BF00302070. [DOI] [PubMed] [Google Scholar]
- 88.Robling AG, Hinant FM, Burr DB, Turner CH. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J Bone Miner Res. 2002;17:1545–1554. doi: 10.1359/jbmr.2002.17.8.1545. [DOI] [PubMed] [Google Scholar]
- 89.Ackerman A, Thornton JC, Wang J, Pierson RNJ, Horlick M. Sex difference in the effect of puberty on the relationship between fat mass and bone mass in 926 healthy subjects, 6 to 18 years old. Obesity (Silver Spring) 2006;14:819–825. doi: 10.1038/oby.2006.95. [DOI] [PubMed] [Google Scholar]
- 90.Arabi A, Tamim H, Nabulsi M, Maalouf J, Khalife H, Choucair M, Vieth R, El-Hajj Fuleihan G. Sex differences in the effect of body-composition variables on bone mass in healthy children and adolescents. Am J Clin Nutr. 2004;80:1428–1435. doi: 10.1093/ajcn/80.5.1428. [DOI] [PubMed] [Google Scholar]
- 91.Ausili E, Rigante D, Salvaggio E, Focarelli B, Rendeli C, Ansuini V, Paolucci V, Triarico S, Martini L, Caradonna P. Determinants of bone mineral density, bone mineral content, and body composition in a cohort of healthy children: influence of sex, age, puberty, and physical activity. Rheumatol Int. 2012;32:2737–2743. doi: 10.1007/s00296-011-2059-8. [DOI] [PubMed] [Google Scholar]
- 92.Pietrobelli A, Faith MS, Wang J, Brambilla P, Chiumello G, Heymsfield SB. Association of lean tissue and fat mass with bone mineral content in children and adolescents. Obes Res. 2002;10:56–60. doi: 10.1038/oby.2002.8. [DOI] [PubMed] [Google Scholar]
- 93.Jackowski SA, Faulkner RA, Farthing JP, Kontulainen SA, Beck TJ, Baxter-Jones AD. Peak lean tissue mass accrual precedes changes in bone strength indices at the proximal femur during the pubertal growth spurt. Bone. 2009;44:1186–1190. doi: 10.1016/j.bone.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 94.Xu L, Nicholson P, Wang Q, Alen M, Cheng S. Bone and muscle development during puberty in girls: a seven-year longitudinal study. J Bone Miner Res. 2009;24:1693–1698. doi: 10.1359/jbmr.090405. [DOI] [PubMed] [Google Scholar]
- 95.Ashby RL, Adams JE, Roberts SA, Mughal MZ, Ward KA. The muscle-bone unit of peripheral and central skeletal sites in children and young adults. Osteoporos Int. 2011;22:121–132. doi: 10.1007/s00198-010-1216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cardel M, Higgins PB, Willig AL, Keita AD, Casazza K, Gower BA, Fernandez JR. African genetic admixture is associated with body composition and fat distribution in a cross-sectional study of children. Int J Obes (Lond) 2011;35:60–65. doi: 10.1038/ijo.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Seeman E, Hopper JL, Bach LA, Cooper ME, Parkinson E, McKay J, Jerums G. Reduced bone mass in daughters of women with osteoporosis. N Engl J Med. 1989;320:554–558. doi: 10.1056/NEJM198903023200903. [DOI] [PubMed] [Google Scholar]
- 98.Soroko SB, Barrett-Connor E, Edelstein SL, Kritz-Silverstein D. Family history of osteoporosis and bone mineral density at the axial skeleton: the Rancho Bernardo Study. J Bone Miner Res. 1994;9:761–769. doi: 10.1002/jbmr.5650090602. [DOI] [PubMed] [Google Scholar]
- 99.Ferrari S, Rizzoli R, Slosman D, Bonjour JP. Familial resemblance for bone mineral mass is expressed before puberty. J Clin Endocrinol Metab. 1998;83:358–361. doi: 10.1210/jcem.83.2.4583. [DOI] [PubMed] [Google Scholar]
- 100.Duren DL, Sherwood RJ, Choh AC, Czerwinski SA, Chumlea WC, Lee M, Sun SS, Demerath EW, Siervogel RM, Towne B. Quantitative genetics of cortical bone mass in healthy 10-year-old children from the Fels Longitudinal Study. Bone. 2007;40:464–470. doi: 10.1016/j.bone.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Estrada K, Styrkarsdottir U, Evangelou E, Hsu YH, Duncan EL, Ntzani EE, Oei L, Albagha OM, Amin N, Kemp JP, Koller DL, Li G, Liu CT, Minster RL, Moayyeri A, Vandenput L, Willner D, Xiao SM, Yerges-Armstrong LM, Zheng HF, Alonso N, Eriksson J, Kammerer CM, Kaptoge SK, Leo PJ, Thorleifsson G, Wilson SG, Wilson JF, Aalto V, Alen M, Aragaki AK, Aspelund T, Center JR, Dailiana Z, Duggan DJ, Garcia M, Garcia-Giralt N, Giroux S, Hallmans G, Hocking LJ, Husted LB, Jameson KA, Khusainova R, Kim GS, Kooperberg C, Koromila T, Kruk M, Laaksonen M, Lacroix AZ, Lee SH, Leung PC, Lewis JR, Masi L, Mencej-Bedrac S, Nguyen TV, Nogues X, Patel MS, Prezelj J, Rose LM, Scollen S, Siggeirsdottir K, Smith AV, Svensson O, Trompet S, Trummer O, van Schoor NM, Woo J, Zhu K, Balcells S, Brandi ML, Buckley BM, Cheng S, Christiansen C, Cooper C, Dedoussis G, Ford I, Frost M, Goltzman D, Gonzalez-Macias J, Kahonen M, Karlsson M, Khusnutdinova E, Koh JM, Kollia P, Langdahl BL, Leslie WD, Lips P, Ljunggren O, Lorenc RS, Marc J, Mellstrom D, Obermayer-Pietsch B, Olmos JM, Pettersson-Kymmer U, Reid DM, Riancho JA, Ridker PM, Rousseau F, Slagboom PE, Tang NL, Urreizti R, Van Hul W, Viikari J, Zarrabeitia MT, Aulchenko YS, Castano-Betancourt M, Grundberg E, Herrera L, Ingvarsson T, Johannsdottir H, Kwan T, Li R, Luben R, Medina-Gomez C, Palsson ST, Reppe S, Rotter JI, Sigurdsson G, van Meurs JB, Verlaan D, Williams FM, Wood AR, Zhou Y, Gautvik KM, Pastinen T, Raychaudhuri S, Cauley JA, Chasman DI, Clark GR, Cummings SR, Danoy P, Dennison EM, Eastell R, Eisman JA, Gudnason V, Hofman A, Jackson RD, Jones G, Jukema JW, Khaw KT, Lehtimaki T, Liu Y, Lorentzon M, McCloskey E, Mitchell BD, Nandakumar K, Nicholson GC, Oostra BA, Peacock M, Pols HA, Prince RL, Raitakari O, Reid IR, Robbins J, Sambrook PN, Sham PC, Shuldiner AR, Tylavsky FA, van Duijn CM, Wareham NJ, Cupples LA, Econs MJ, Evans DM, Harris TB, Kung AW, Psaty BM, Reeve J, Spector TD, Streeten EA, Zillikens MC, Thorsteinsdottir U, Ohlsson C, Karasik D, Richards JB, Brown MA, Stefansson K, Uitterlinden AG, Ralston SH, Ioannidis JP, Kiel DP, Rivadeneira F. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44:491–501. doi: 10.1038/ng.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zheng HF, Tobias JH, Duncan E, Evans DM, Eriksson J, Paternoster L, Yerges-Armstrong LM, Lehtimaki T, Bergstrom U, Kahonen M, Leo PJ, Raitakari O, Laaksonen M, Nicholson GC, Viikari J, Ladouceur M, Lyytikainen LP, Medina-Gomez C, Rivadeneira F, Prince RL, Sievanen H, Leslie WD, Mellstrom D, Eisman JA, Moverare-Skrtic S, Goltzman D, Hanley DA, Jones G, St Pourcain B, Xiao Y, Timpson NJ, Smith GD, Reid IR, Ring SM, Sambrook PN, Karlsson M, Dennison EM, Kemp JP, Danoy P, Sayers A, Wilson SG, Nethander M, McCloskey E, Vandenput L, Eastell R, Liu J, Spector T, Mitchell BD, Streeten EA, Brommage R, Pettersson-Kymmer U, Brown MA, Ohlsson C, Richards JB, Lorentzon M. WNT16 influences bone mineral density, cortical bone thickness, bone strength, and osteoporotic fracture risk. PLoS Genet. 2012;8:e1002745. doi: 10.1371/journal.pgen.1002745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mora S, Gilsanz V. Establishment of peak bone mass. Endocrinol Metab Clin North Am. 2003;32:39–63. doi: 10.1016/s0889-8529(02)00058-0. [DOI] [PubMed] [Google Scholar]
- 104.Krall EA, Dawson-Hughes B. Heritable and life-style determinants of bone mineral density. J Bone Miner Res. 1993;8:1–9. doi: 10.1002/jbmr.5650080102. [DOI] [PubMed] [Google Scholar]
- 105.Chevalley T, Rizzoli R, Hans D, Ferrari S, Bonjour JP. Interaction between calcium intake and menarcheal age on bone mass gain: an eight-year follow-up study from prepuberty to postmenarche. J Clin Endocrinol Metab. 2005;90:44–51. doi: 10.1210/jc.2004-1043. [DOI] [PubMed] [Google Scholar]
- 106.Timpson NJ, Tobias JH, Richards JB, Soranzo N, Duncan EL, Sims AM, Whittaker P, Kumanduri V, Zhai G, Glaser B, Eisman J, Jones G, Nicholson G, Prince R, Seeman E, Spector TD, Brown MA, Peltonen L, Smith GD, Deloukas P, Evans DM. Common variants in the region around Osterix are associated with bone mineral density and growth in childhood. Hum Mol Genet. 2009;18:1510–1517. doi: 10.1093/hmg/ddp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gueguen R, Jouanny P, Guillemin F, Kuntz C, Pourel J, Siest G. Segregation analysis and variance components analysis of bone mineral density in healthy families. J Bone Miner Res. 1995;10:2017–2022. doi: 10.1002/jbmr.5650101223. [DOI] [PubMed] [Google Scholar]
- 108.McCormick DP, Ponder SW, Fawcett HD, Palmer JL. Spinal bone mineral density in 335 normal and obese children and adolescents: evidence for ethnic and sex differences. J Bone Miner Res. 1991;6:507–513. doi: 10.1002/jbmr.5650060513. [DOI] [PubMed] [Google Scholar]
- 109.Bachrach LK, Hastie T, Wang MC, Narasimhan B, Marcus R. Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: a longitudinal study. J Clin Endocrinol Metab. 1999;84:4702–4712. doi: 10.1210/jcem.84.12.6182. [DOI] [PubMed] [Google Scholar]
- 110.Bhudhikanok GS, Wang MC, Eckert K, Matkin C, Marcus R, Bachrach LK. Differences in bone mineral in young Asian and Caucasian Americans may reflect differences in bone size. J Bone Miner Res. 1996;11:1545–1556. doi: 10.1002/jbmr.5650111023. [DOI] [PubMed] [Google Scholar]
- 111.Armstrong GT, Chow EJ, Sklar CA. Alterations in pubertal timing following therapy for childhood malignancies. Endocr Dev. 2009;15:25–39. doi: 10.1159/000207616. [DOI] [PubMed] [Google Scholar]
- 112.Weaver CM, McCabe LD, McCabe GP, Novotny R, Van Loan M, Going S, Matkovic V, Boushey C, Savaiano DA. Bone mineral and predictors of bone mass in white, Hispanic, and Asian early pubertal girls. Calcif Tissue Int. 2007;81:352–363. doi: 10.1007/s00223-007-9074-5. [DOI] [PubMed] [Google Scholar]
- 113.Gilsanz V, Kovanlikaya A, Costin G, Roe TF, Sayre J, Kaufman F. Differential effect of gender on the sizes of the bones in the axial and appendicular skeletons. J Clin Endocrinol Metab. 1997;82:1603–1607. doi: 10.1210/jcem.82.5.3942. [DOI] [PubMed] [Google Scholar]
- 114.Gilsanz V. Bone density in children: a review of the available techniques and indications. Eur J Radiol. 1998;26:177–182. doi: 10.1016/s0720-048x(97)00093-4. [DOI] [PubMed] [Google Scholar]
- 115.Leonard MB, Elmi A, Mostoufi-Moab S, Shults J, Burnham JM, Thayu M, Kibe L, Wetzsteon RJ, Zemel BS. Effects of sex, race, and puberty on cortical bone and the functional muscle bone unit in children, adolescents, and young adults. J Clin Endocrinol Metab. 2010;95:1681–1689. doi: 10.1210/jc.2009-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ellis KJ. Body composition of a young, multiethnic, male population. Am J Clin Nutr. 1997;66:1323–1331. doi: 10.1093/ajcn/66.6.1323. [DOI] [PubMed] [Google Scholar]
- 117.Ellis KJ, Abrams SA, Wong WW. Body composition of a young, muiltiethnic female population. Am J Clin Nutr. 1997;65:724–731. doi: 10.1093/ajcn/65.3.724. [DOI] [PubMed] [Google Scholar]
- 118.Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, Mahboubi S, Fan B, Frederick MM, Winer K, Shepherd JA. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab. 2007;92:2087–2099. doi: 10.1210/jc.2006-2553. [DOI] [PubMed] [Google Scholar]
- 119.Hammami M, Koo WW, Hockman EM. Body composition of neonates from fan beam dual energy X-ray absorptiometry measurement. JPEN J Parenter Enteral Nutr. 2003;27:423–426. doi: 10.1177/0148607103027006423. [DOI] [PubMed] [Google Scholar]
- 120.Koo WW, Bush AJ, Walters J, Carlson SE. Postnatal development of bone mineral status during infancy. J Am Coll Nutr. 1998;17:65–70. doi: 10.1080/07315724.1998.10720457. [DOI] [PubMed] [Google Scholar]
- 121.Kurl S, Heinonen K, Jurvelin JS, Lansimies E. Lumbar bone mineral content and density measured using a Lunar DPX densitometer in healthy full-term infants during the first year of life. Clin Physiol Func Imaging. 2002;22:222–225. doi: 10.1046/j.1475-097x.2002.00422.x. [DOI] [PubMed] [Google Scholar]
- 122.Unal A, Gur E, Arvas A, Erginel A, AlikasifoAglu M, Ilter O. Bone density values in healthy Turkish infants. Indian Pediatr. 2000;37:497–503. [PubMed] [Google Scholar]
- 123.Rupich RC, Specker BL, Lieuw AFM, Ho M. Gender and race differences in bone mass during infancy. Calcif Tissue Int. 1996;58:395–397. doi: 10.1007/BF02509436. [DOI] [PubMed] [Google Scholar]
- 124.Willing MC, Torner JC, Burns TL, Janz KF, Marshall TA, Gilmore J, Warren JJ, Levy SM. Percentile distributions of bone measurements in Iowa children: the Iowa Bone Development Study. J Clin Densitom. 2005;8:39–47. doi: 10.1385/jcd:8:1:039. [DOI] [PubMed] [Google Scholar]
- 125.Kontulainen SA, Macdonald HM, Khan KM, McKay HA. Examining bone surfaces across puberty: a 20-month pQCT trial. J Bone Miner Res. 2005;20:1202–1207. doi: 10.1359/JBMR.050214. [DOI] [PubMed] [Google Scholar]
- 126.Macdonald H, Kontulainen S, Petit M, Janssen P, McKay H. Bone strength and its determinants in pre- and early pubertal boys and girls. Bone. 2006;39:598–608. doi: 10.1016/j.bone.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 127.Nishiyama KK, Macdonald HM, Moore SA, Fung T, Boyd SK, McKay HA. Cortical porosity is higher in boys compared with girls at the distal radius and distal tibia during pubertal growth: an HR-pQCT study. J Bone Miner Res. 2012;27:273–282. doi: 10.1002/jbmr.552. [DOI] [PubMed] [Google Scholar]
- 128.Gilsanz V, Chalfant J, Kalkwarf H, Zemel B, Lappe J, Oberfield S, Shepherd J, Wren T, Winer K. Age at onset of puberty predicts bone mass in young adulthood. J Pediatr. 2011;158:100–105. doi: 10.1016/j.jpeds.2010.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chevalley T, Bonjour JP, Ferrari S, Rizzoli R. The influence of pubertal timing on bone mass acquisition: a predetermined trajectory detectable five years before menarche. J Clin Endocrinol Metab. 2009;94:3424–3431. doi: 10.1210/jc.2009-0241. [DOI] [PubMed] [Google Scholar]
- 130.Jackowski SA, Erlandson MC, Mirwald RL, Faulkner RA, Bailey DA, Kontulainen SA, Cooper DM, Baxter-Jones AD. Effect of maturational timing on bone mineral content accrual from childhood to adulthood: evidence from 15 years of longitudinal data. Bone. 2011;48:1178–1185. doi: 10.1016/j.bone.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 131.Darelid A, Ohlsson C, Nilsson M, Kindblom JM, Mellstrom D, Lorentzon M. Catch up in bone acquisition in young adult men with late normal puberty. J Bone Miner Res. 2012;27:2198–2207. doi: 10.1002/jbmr.1675. [DOI] [PubMed] [Google Scholar]
- 132.Nikander R, Sievanen H, Heinonen A, Daly RM, Uusi-Rasi K, Kannus P. Targeted exercise against osteoporosis: a systematic review and meta-analysis for optimising bone strength throughout life. BMC Med. 2010;8:47. doi: 10.1186/1741-7015-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ishikawa S, Kim Y, Kang M, Morgan DW. Effects of weight-bearing exercise on bone health in girls: a meta-analysis. Sports Med. 2013;43:875–892. doi: 10.1007/s40279-013-0060-y. [DOI] [PubMed] [Google Scholar]
- 134.Burt LA, Greene DA, Ducher G, Naughton GA. Skeletal adaptations associated with pre-pubertal gymnastics participation as determined by DXA and pQCT: a systematic review and meta-analysis. J Sci Med Sport. 2013;16:231–239. doi: 10.1016/j.jsams.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 135.Behringer M, Gruetzner S, McCourt M, Mester J. Effects of weight-bearing activities on bone mineral content and density in children and adolescents: a meta-analysis. J Bone Miner Res. 2014;29:467–478. doi: 10.1002/jbmr.2036. [DOI] [PubMed] [Google Scholar]
- 136.Nogueira RC, Weeks BK, Beck BR. Exercise to improve pediatric bone and fat: a systematic review and meta-analysis. Med Sci Sports Exerc. 2014;46:610–621. doi: 10.1249/MSS.0b013e3182a6ab0d. [DOI] [PubMed] [Google Scholar]
- 137.Tan VP, Macdonald HM, Kim S, Nettlefold L, Gabel L, Ashe MC, McKay HA. Influence of physical activity on bone strength in children and adolescents: a systematic review and narrative synthesis. J Bone Miner Res. 2014;29:2161–2181. doi: 10.1002/jbmr.2254. [DOI] [PubMed] [Google Scholar]
- 138.Cho SS, Qi L, Fahey GCJ, Klurfeld DM. Consumption of cereal fiber, mixtures of whole grains and bran, and whole grains and risk reduction in type 2 diabetes, obesity, and cardiovascular disease. Am J Clin Nutr. 2013;98:594–619. doi: 10.3945/ajcn.113.067629. [DOI] [PubMed] [Google Scholar]
- 139.American Diabetes Association Introduction: the American Diabetes Association’s (ADA) evidence-based practice guidelines, standards, and related recommendations and documents for diabetes care. Diabetes Care. 2012;35:S1–S2. doi: 10.2337/dc12-s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Woolf SH. Weighing the evidence to formulate dietary guidelines. J Am Coll Nutr. 2006;25:277S–284S. doi: 10.1080/07315724.2006.10719578. [DOI] [PubMed] [Google Scholar]
- 141.Hogstrom M, Nordstrom P, Nordstrom A. n-3 Fatty acids are positively associated with peak bone mineral density and bone accrual in healthy men: the NO2 Study. Am J Clin Nutr. 2007;85:803–807. doi: 10.1093/ajcn/85.3.803. [DOI] [PubMed] [Google Scholar]
- 142.Eriksson S, Mellstrom D, Strandvik B. Fatty acid pattern in serum is associated with bone mineralisation in healthy 8-year-old children. Br J Nutr. 2009;102:407–412. doi: 10.1017/S0007114508190286. [DOI] [PubMed] [Google Scholar]
- 143.Ballard TL, Specker BL, Binkley TL, Vukovich MD. Effect of protein supplementation during a 6-month strength and conditioning program on areal and volumetric bone parameters. Bone. 2006;38:898–904. doi: 10.1016/j.bone.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 144.Alexy U, Remer T, Manz F, Neu CM, Schoenau E. Long-term protein intake and dietary potential renal acid load are associated with bone modeling and remodeling at the proximal radius in healthy children. Am J Clin Nutr. 2005;82:1107–1114. doi: 10.1093/ajcn/82.5.1107. [DOI] [PubMed] [Google Scholar]
- 145.Remer T, Manz F, Alexy U, Schoenau E, Wudy SA, Shi L. Long-term high urinary potential renal acid load and low nitrogen excretion predict reduced diaphyseal bone mass and bone size in children. J Clin Endocrinol Metab. 2011;96:2861–2868. doi: 10.1210/jc.2011-1005. [DOI] [PubMed] [Google Scholar]
- 146.Bounds W, Skinner J, Carruth BR, Ziegler P. The relationship of dietary and lifestyle factors to bone mineral indexes in children. J Am Diet Assoc. 2005;105:735–741. doi: 10.1016/j.jada.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 147.Vatanparast H, Bailey DA, Baxter-Jones AD, Whiting SJ. The effects of dietary protein on bone mineral mass in young adults may be modulated by adolescent calcium intake. J Nutr. 2007;137:2674–2679. doi: 10.1093/jn/137.12.2674. [DOI] [PubMed] [Google Scholar]
- 148.Zhang Q, Ma G, Greenfield H, Zhu K, Du X, Foo LH, Hu X, Fraser DR. The association between dietary protein intake and bone mass accretion in pubertal girls with low calcium intakes. Br J Nutr. 2010;103:714–723. doi: 10.1017/S0007114509992303. [DOI] [PubMed] [Google Scholar]
- 149.Hoppe C, Molgaard C, Michaelsen KF. Bone size and bone mass in 10-year-old Danish children: effect of current diet. Osteoporos Int. 2000;11:1024–1030. doi: 10.1007/s001980070023. [DOI] [PubMed] [Google Scholar]
- 150.Iuliano-Burns S, Stone J, Hopper JL, Seeman E. Diet and exercise during growth have site-specific skeletal effects: a co-twin control study. Osteoporos Int. 2005;16:1225–1232. doi: 10.1007/s00198-004-1830-z. [DOI] [PubMed] [Google Scholar]
- 151.Chevalley T, Bonjour JP, Ferrari S, Rizzoli R. High-protein intake enhances the positive impact of physical activity on BMC in prepubertal boys. J Bone Miner Res. 2008;23:131–142. doi: 10.1359/jbmr.070907. [DOI] [PubMed] [Google Scholar]
- 152.Esterle L, Sabatier JP, Guillon-Metz F, Walrant-Debray O, Guaydier-Souquieres G, Jehan F, Garabedian M. Milk, rather than other foods, is associated with vertebral bone mass and circulating IGF-1 in female adolescents. Osteoporos Int. 2009;20:567–575. doi: 10.1007/s00198-008-0708-x. [DOI] [PubMed] [Google Scholar]
- 153.Ekbote VH, Khadilkar AV, Chiplonkar SA, Khadilkar VV. Determinants of bone mineral content and bone area in Indian preschool children. J Bone Miner Metab. 2011;29:334–341. doi: 10.1007/s00774-010-0224-x. [DOI] [PubMed] [Google Scholar]
- 154.Libuda L, Wudy SA, Schoenau E, Remer T. Comparison of the effects of dietary protein, androstenediol and forearm muscle area on radial bone variables in healthy prepubertal children. Br J Nutr. 2011;105:428–435. doi: 10.1017/S0007114510003508. [DOI] [PubMed] [Google Scholar]
- 155.Iuliano-Burns S, Saxon L, Naughton G, Gibbons K, Bass SL. Regional specificity of exercise and calcium during skeletal growth in girls: a randomized controlled trial. J Bone Miner Res. 2003;18:156–162. doi: 10.1359/jbmr.2003.18.1.156. [DOI] [PubMed] [Google Scholar]
- 156.Stear SJ, Prentice A, Jones SC, Cole TJ. Effect of a calcium and exercise intervention on the bone mineral status of 16–18-y-old adolescent girls. Am J Clin Nutr. 2003;77:985–992. doi: 10.1093/ajcn/77.4.985. [DOI] [PubMed] [Google Scholar]
- 157.Bass SL, Naughton G, Saxon L, Iuliano-Burns S, Daly R, Briganti EM, Hume C, Nowson C. Exercise and calcium combined results in a greater osteogenic effect than either factor alone: a blinded randomized placebo-controlled trial in boys. J Bone Miner Res. 2007;22:458–464. doi: 10.1359/jbmr.061201. [DOI] [PubMed] [Google Scholar]
- 158.Courteix D, Jaffre C, Lespessailles E, Benhamou L. Cumulative effects of calcium supplementation and physical activity on bone accretion in premenarchal children: a double-blind randomised placebo-controlled trial. Int J Sports Med. 2005;26:332–338. doi: 10.1055/s-2004-821040. [DOI] [PubMed] [Google Scholar]
- 159.Cheng S, Lyytikainen A, Kroger H, Lamberg-Allardt C, Alen M, Koistinen A, Wang QJ, Suuriniemi M, Suominen H, Mahonen A, Nicholson PH, Ivaska KK, Korpela R, Ohlsson C, Vaananen KH, Tylavsky F. Effects of calcium, dairy product, and vitamin D supplementation on bone mass accrual and body composition in 10–12-y-old girls: a 2-y randomized trial. Am J Clin Nutr. 2005;82:1115–1126. doi: 10.1093/ajcn/82.5.1115. [DOI] [PubMed] [Google Scholar]
- 160.Dibba B, Prentice A, Ceesay M, Stirling DM, Cole TJ, Poskitt EM. Effect of calcium supplementation on bone mineral accretion in Gambian children accustomed to a low-calcium diet. Am J Clin Nutr. 2000;71:544–549. doi: 10.1093/ajcn/71.2.544. [DOI] [PubMed] [Google Scholar]
- 161.Cameron MA, Paton LM, Nowson CA, Margerison C, Frame M, Wark JD. The effect of calcium supplementation on bone density in premenarcheal females: a co-twin approach. J Clin Endocrinol Metab. 2004;89:4916–4922. doi: 10.1210/jc.2003-031985. [DOI] [PubMed] [Google Scholar]
- 162.Prentice A, Ginty F, Stear SJ, Jones SC, Laskey MA, Cole TJ. Calcium supplementation increases stature and bone mineral mass of 16- to 18-year-old boys. J Clin Endocrinol Metab. 2005;90:3153–3161. doi: 10.1210/jc.2004-2114. [DOI] [PubMed] [Google Scholar]
- 163.Moyer-Mileur LJ, Xie B, Ball SD, Pratt T. Bone mass and density response to a 12-month trial of calcium and vitamin D supplement in preadolescent girls. J Musculoskelet Neuronal Interact. 2003;3:63–70. [PubMed] [Google Scholar]
- 164.Greene DA, Naughton GA. Calcium and vitamin-D supplementation on bone structural properties in peripubertal female identical twins: a randomised controlled trial. Osteoporos Int. 2011;22:489–498. doi: 10.1007/s00198-010-1317-z. [DOI] [PubMed] [Google Scholar]
- 165.Lambert HL, Eastell R, Karnik K, Russell JM, Barker ME. Calcium supplementation and bone mineral accretion in adolescent girls: an 18-mo randomized controlled trial with 2-y follow-up. Am J Clin Nutr. 2008;87:455–462. doi: 10.1093/ajcn/87.2.455. [DOI] [PubMed] [Google Scholar]
- 166.Gibbons MJ, Gilchrist NL, Frampton C, Maguire P, Reilly PH, March RL, Wall CR. The effects of a high calcium dairy food on bone health in pre-pubertal children in New Zealand. Asia Pac J Clin Nutr. 2004;13:341–347. [PubMed] [Google Scholar]
- 167.Ho SC, Guldan GS, Woo J, Yu R, Tse MM, Sham A, Cheng J. A prospective study of the effects of 1-year calcium-fortified soy milk supplementation on dietary calcium intake and bone health in Chinese adolescent girls aged 14 to 16. Osteoporos Int. 2005;16:1907–1916. doi: 10.1007/s00198-005-1963-8. [DOI] [PubMed] [Google Scholar]
- 168.Chevalley T, Bonjour JP, Ferrari S, Hans D, Rizzoli R. Skeletal site selectivity in the effects of calcium supplementation on areal bone mineral density gain: a randomized, double-blind, placebo-controlled trial in prepubertal boys. J Clin Endocrinol Metab. 2005;90:3342–3349. doi: 10.1210/jc.2004-1455. [DOI] [PubMed] [Google Scholar]
- 169.Merrilees MJ, Smart EJ, Gilchrist NL, Frampton C, Turner JG, Hooke E, March RL, Maguire P. Effects of dairy food supplements on bone mineral density in teenage girls. Eur J Nutr. 2000;39:256–262. doi: 10.1007/s003940070004. [DOI] [PubMed] [Google Scholar]
- 170.Du X, Zhu K, Trube A, Zhang Q, Ma G, Hu X, Fraser DR, Greenfield H. School-milk intervention trial enhances growth and bone mineral accretion in Chinese girls aged 10–12 years in Beijing. Br J Nutr. 2004;92:159–168. doi: 10.1079/BJN20041118. [DOI] [PubMed] [Google Scholar]
- 171.Carter LM, Whiting SJ, Drinkwater DT, Zello GA, Faulkner RA, Bailey DA. Self-reported calcium intake and bone mineral content in children and adolescents. J Am Coll Nutr. 2001;20:502–509. doi: 10.1080/07315724.2001.10719059. [DOI] [PubMed] [Google Scholar]
- 172.Lappe JM, Watson P, Gilsanz V, Hangartner T, Kalkwarf HJ, Oberfield S, Shepherd J, Winer KK, Zemel B. The longitudinal effects of physical activity and dietary calcium on bone mass accrual across stages of pubertal development. J Bone Miner Res. 2015;30:156–164. doi: 10.1002/jbmr.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Molgaard C, Larnkjaer A, Cashman KD, Lamberg-Allardt C, Jakobsen J, Michaelsen KF. Does vitamin D supplementation of healthy Danish Caucasian girls affect bone turnover and bone mineralization? Bone. 2010;46:432–439. doi: 10.1016/j.bone.2009.08.056. [DOI] [PubMed] [Google Scholar]
- 174.Lloyd T, Chinchilli VM, Johnson-Rollings N, Kieselhorst K, Eggli DF, Marcus R. Adult female hip bone density reflects teenage sports-exercise patterns but not teenage calcium intake. Pediatrics. 2000;106:40–44. doi: 10.1542/peds.106.1.40. [DOI] [PubMed] [Google Scholar]
- 175.El-Hajj Fuleihan G, Nabulsi M, Tamim H, Maalouf J, Salamoun M, Khalife H, Choucair M, Arabi A, Vieth R. Effect of vitamin D replacement on musculoskeletal parameters in school children: a randomized controlled trial. J Clin Endocrinol Metab. 2006;91:405–412. doi: 10.1210/jc.2005-1436. [DOI] [PubMed] [Google Scholar]
- 176.Al-Shaar L, Nabulsi M, Maalouf J, El-Rassi R, Vieth R, Beck TJ, El-Hajj Fuleihan G. Effect of vitamin D replacement on hip structural geometry in adolescents: a randomized controlled trial. Bone. 2013;56:296–303. doi: 10.1016/j.bone.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 177.Viljakainen HT, Natri AM, Karkkainen M, Huttunen MM, Palssa A, Jakobsen J, Cashman KD, Molgaard C, Lamberg-Allardt C. A positive dose–response effect of vitamin D supplementation on site-specific bone mineral augmentation in adolescent girls: a double-blinded randomized placebo-controlled 1-year intervention. J Bone Miner Res. 2006;21:836–844. doi: 10.1359/jbmr.060302. [DOI] [PubMed] [Google Scholar]
- 178.Khadilkar AV, Sayyad MG, Sanwalka NJ, Bhandari DR, Naik S, Khadilkar VV, Mughal MZ. Vitamin D supplementation and bone mass accrual in underprivileged adolescent Indian girls. Asia Pac J Clin Nutr. 2010;19:465–472. [PubMed] [Google Scholar]
- 179.Ward KA, Das G, Roberts SA, Berry JL, Adams JE, Rawer R, Mughal MZ. A randomized, controlled trial of vitamin D supplementation upon musculoskeletal health in postmenarchal females. J Clin Endocrinol Metab. 2010;95:4643–4651. doi: 10.1210/jc.2009-2725. [DOI] [PubMed] [Google Scholar]
- 180.Andersen R, Molgaard C, Skovgaard LT, Brot C, Cashman KD, Jakobsen J, Lamberg-Allardt C, Ovesen L. Effect of vitamin D supplementation on bone and vitamin D status among Pakistani immigrants in Denmark: a randomised double-blinded placebo-controlled intervention study. Br J Nutr. 2008;100:197–207. doi: 10.1017/S000711450789430X. [DOI] [PubMed] [Google Scholar]
- 181.Breen ME, Laing EM, Hall DB, Hausman DB, Taylor RG, Isales CM, Ding KH, Pollock NK, Hamrick MW, Baile CA, Lewis RD. 25-hydroxyvitamin D, insulin-like growth factor-I, and bone mineral accrual during growth. J Clin Endocrinol Metab. 2011;96:E89–E98. doi: 10.1210/jc.2010-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cheng S, Tylavsky F, Kröger H, Kärkkäinen M, Lyytikäinen A, Koistinen A, Mahonen A, Alen M, Halleen J, Väänänen K, Lamberg-Allardt C. Association of low 25-hydroxyvitamin D concentrations with elevated parathyroid hormone concentrations and low cortical bone density in early pubertal and prepubertal Finnish girls. Am J Clin Nutr. 2003;78:485–492. doi: 10.1093/ajcn/78.3.485. [DOI] [PubMed] [Google Scholar]
- 183.Foo LH, Zhang Q, Zhu K, Ma G, Hu X, Greenfield H, Fraser DR. Low vitamin D status has an adverse influence on bone mass, bone turnover, and muscle strength in Chinese adolescent girls. J Nutr. 2009;139:1002–1007. doi: 10.3945/jn.108.102053. [DOI] [PubMed] [Google Scholar]
- 184.Lee YA, Kim JY, Kang MJ, Chung SJ, Shin CH, Yang SW. Adequate vitamin D status and adiposity contribute to bone health in peripubertal nonobese children. J Bone Miner Metab. 2013;31:337–345. doi: 10.1007/s00774-012-0419-4. [DOI] [PubMed] [Google Scholar]
- 185.Carpenter TO, DeLucia MC, Zhang JH, Bejnerowicz G, Tartamella L, Dziura J, Petersen KF, Befroy D, Cohen D. A randomized controlled study of effects of dietary magnesium oxide supplementation on bone mineral content in healthy girls. J Clin Endocrinol Metab. 2006;91:4866–4872. doi: 10.1210/jc.2006-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Grobler SR, Louw AJ, Chikte UM, Rossouw RJ, van W Kotze TJ. The relationships between two different drinking water fluoride levels, dental fluorosis and bone mineral density of children. Open Dent J. 2009;3:48–54. doi: 10.2174/1874210600903010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Levy SM, Eichenberger-Gilmore J, Warren JJ, Letuchy E, Broffitt B, Marshall TA, Burns T, Willing M, Janz K, Torner JC. Associations of fluoride intake with children’s bone measures at age 11. Community Dent Oral Epidemiol. 2009;37:416–426. doi: 10.1111/j.1600-0528.2009.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Levy SM, Warren JJ, Phipps K, Letuchy E, Broffitt B, Eichenberger-Gilmore J, Burns TL, Kavand G, Janz KF, Torner JC, Pauley CA. Effects of life-long fluoride intake on bone measures of adolescents: a prospective cohort study. J Dent Res. 2014;93:353–359. doi: 10.1177/0022034514520708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Prynne CJ, Mishra GD, O’Connell MA, Muniz G, Laskey MA, Yan L, Prentice A, Ginty F. Fruit and vegetable intakes and bone mineral status: a cross sectional study in 5 age and sex cohorts. Am J Clin Nutr. 2006;83:1420–1428. doi: 10.1093/ajcn/83.6.1420. [DOI] [PubMed] [Google Scholar]
- 190.Laudermilk M, Manore M, Thomson C, Houtkooper L, Farr J, Going S. Vitamin C and zinc intakes are related to bone macroarchitectural structure and strength in prepubescent girls. Calcif Tissue Int. 2012;91:430–439. doi: 10.1007/s00223-012-9656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.O’Connor E, Molgaard C, Michaelsen KF, Jakobsen J, Lamberg-Allardt CJ, Cashman KD. Serum percentage undercarboxylated osteocalcin, a sensitive measure of vitamin K status, and its relationship to bone health indices in Danish girls. Br J Nutr. 2007;97:661–666. doi: 10.1017/S0007114507433050. [DOI] [PubMed] [Google Scholar]
- 192.Kalkwarf HJ, Khoury JC, Bean J, Elliot JG. Vitamin K, bone turnover, and bone mass in girls. Am J Clin Nutr. 2004;80:1075–1080. doi: 10.1093/ajcn/80.4.1075. [DOI] [PubMed] [Google Scholar]
- 193.Abrams SA, Griffin IJ, Hawthorne KM, Liang L, Gunn SK, Darlington G, Ellis KJ. A combination of prebiotic short- and long-chain inulin-type fructans enhances calcium absorption and bone mineralization in young adolescents. Am J Clin Nutr. 2005;82:471–476. doi: 10.1093/ajcn.82.2.471. [DOI] [PubMed] [Google Scholar]
- 194.McGartland C, Robson PJ, Murray L, Cran G, Savage MJ, Watkins D, Rooney M, Boreham C. Carbonated soft drink consumption and bone mineral density in adolescence: the Northern Ireland Young Hearts project. J Bone Miner Res. 2003;18:1563–1569. doi: 10.1359/jbmr.2003.18.9.1563. [DOI] [PubMed] [Google Scholar]
- 195.McGartland CP, Robson PJ, Murray LJ, Cran GW, Savage MJ, Watkins DC, Rooney MM, Boreham CA. Fruit and vegetable consumption and bone mineral density: the Northern Ireland Young Hearts Project. Am J Clin Nutr. 2004;80:1019–1023. doi: 10.1093/ajcn/80.4.1019. [DOI] [PubMed] [Google Scholar]
- 196.Tylavsky FA, Holliday K, Danish R, Womack C, Norwood J, Carbone L. Fruit and vegetable intakes are an independent predictor of bone size in early pubertal children. Am J Clin Nutr. 2004;79:311–317. doi: 10.1093/ajcn/79.2.311. [DOI] [PubMed] [Google Scholar]
- 197.Whiting SJ, Vatanparast H, Baxter-Jones A, Faulkner RA, Mirwald R, Bailey DA. Factors that affect bone mineral accrual in the adolescent growth spurt. J Nutr. 2004;134:696S–700S. doi: 10.1093/jn/134.3.696S. [DOI] [PubMed] [Google Scholar]
- 198.Vatanparast H, Baxter-Jones A, Faulkner RA, Bailey DA, Whiting SJ. Positive effects of vegetable and fruit consumption and calcium intake on bone mineral accrual in boys during growth from childhood to adolescence: the University of Saskatchewan Pediatric Bone Mineral Accrual Study. Am J Clin Nutr. 2005;82:700–706. doi: 10.1093/ajcn.82.3.700. [DOI] [PubMed] [Google Scholar]
- 199.Ma D, Jones G. Soft drink and milk consumption, physical activity, bone mass, and upper limb fractures in children: a population-based case–control study. Calcif Tissue Int. 2004;75:286–291. doi: 10.1007/s00223-004-0274-y. [DOI] [PubMed] [Google Scholar]
- 200.Wyshak G. Teenaged girls, carbonated beverage consumption, and bone fractures. Arch Pediatr Adolesc Med. 2000;154:610–613. doi: 10.1001/archpedi.154.6.610. [DOI] [PubMed] [Google Scholar]
- 201.Manias K, McCabe D, Bishop N. Fractures and recurrent fractures in children; varying effects of environmental factors as well as bone size and mass. Bone. 2006;39:652–657. doi: 10.1016/j.bone.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 202.Libuda L, Alexy U, Remer T, Stehle P, Schoenau E, Kersting M. Association between long-term consumption of soft drinks and variables of bone modeling and remodeling in a sample of healthy German children and adolescents. Am J Clin Nutr. 2008;88:1670–1677. doi: 10.3945/ajcn.2008.26414. [DOI] [PubMed] [Google Scholar]
- 203.Hasling C, Søndergaard K, Charles P, Mosekilde L. Calcium metabolism in postmenopausal osteoporotic women is determined by dietary calcium and coffee intake. J Nutr. 1992;122:1119–1126. doi: 10.1093/jn/122.5.1119. [DOI] [PubMed] [Google Scholar]
- 204.Conlisk AJ, Galuska DA. Is caffeine associated with bone mineral density in young adult women? Prev Med. 2000;31:562–568. doi: 10.1006/pmed.2000.0742. [DOI] [PubMed] [Google Scholar]
- 205.Ho-Pham LT, Nguyen ND, Nguyen TV. Effect of vegetarian diets on bone mineral density: a Bayesian meta-analysis. Am J Clin Nutr. 2009;90:943–950. doi: 10.3945/ajcn.2009.27521. [DOI] [PubMed] [Google Scholar]
- 206.Appleby P, Roddam A, Allen N, Key T. Comparative fracture risk in vegetarians and nonvegetarians in EPIC-Oxford. Eur J Clin Nutr. 2007;61:1400–1406. doi: 10.1038/sj.ejcn.1602659. [DOI] [PubMed] [Google Scholar]
- 207.Weaver CM, Hill KM. Osteoporosis: the early years. In: Coulston AM, Boushey CJ, Ferruzzi MG, editors. Nutrition in the prevention and treatment of disease. 3. San Diego: Elsevier Inc.; 2013. pp. 839–858. [Google Scholar]
- 208.Weaver CM. Role of dairy beverages in the diet. Physiol Behav. 2010;100:63–66. doi: 10.1016/j.physbeh.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 209.Wallace TC, Reider C, Fulgoni VL., 3rd Calcium and vitamin D disparities are related to gender, age, race, household income level, and weight classification but not vegetarian status in the United States: analysis of the NHANES 2001–2008 data set. J Am Coll Nutr. 2013;32:321–330. doi: 10.1080/07315724.2013.839905. [DOI] [PubMed] [Google Scholar]
- 210.Lanham-New SA. Is “vegetarianism” a serious risk factor for osteoporotic fracture? Am J Clin Nutr. 2009;90:910–911. doi: 10.3945/ajcn.2009.28542. [DOI] [PubMed] [Google Scholar]
- 211.Koo WW, Hammami M, Margeson DP, Nwaesei C, Montalto MB, Lasekan JB. Reduced bone mineralization in infants fed palm olein-containing formula: a randomized, double-blinded, prospective trial. Pediatrics. 2003;111:1017–1023. doi: 10.1542/peds.111.5.1017. [DOI] [PubMed] [Google Scholar]
- 212.Molgaard C, Larnkjaer A, Mark AB, Michaelsen KF. Are early growth and nutrition related to bone health in adolescence? The Copenhagen Cohort Study of infant nutrition and growth. Am J Clin Nutr. 2011;94:1865S–1869S. doi: 10.3945/ajcn.110.001214. [DOI] [PubMed] [Google Scholar]
- 213.Harvey NC, Robinson SM, Crozier SR, Marriott LD, Gale CR, Cole ZA, Inskip HM, Godfrey KM, Cooper C. Breast-feeding and adherence to infant feeding guidelines do not influence bone mass at age 4 years. Br J Nutr. 2009;102:915–920. doi: 10.1017/S0007114509317420. [DOI] [PubMed] [Google Scholar]
- 214.Pirila S, Taskinen M, Viljakainen H, Kajosaari M, Turanlahti M, Saarinen-Pihkala UM, Makitie O. Infant milk feeding influences adult bone health: a prospective study from birth to 32 years. PLoS One. 2011;6:e19068. doi: 10.1371/journal.pone.0019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kalkwarf HJ, Zemel BS, Yolton K, Heubi JE. Bone mineral content and density of the lumbar spine of infants and toddlers: influence of age, sex, race, growth, and human milk feeding. J Bone Miner Res. 2013;28:206–212. doi: 10.1002/jbmr.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Butte NF, Wong WW, Hopkinson JM, Smith EO, Ellis KJ. Infant feeding mode affects early growth and body composition. Pediatrics. 2000;106:1355–1366. doi: 10.1542/peds.106.6.1355. [DOI] [PubMed] [Google Scholar]
- 217.Jones G, Riley M, Dwyer T. Breastfeeding in early life and bone mass in prepubertal children: a longitudinal study. Osteoporos Int. 2000;11:146–152. doi: 10.1007/PL00004176. [DOI] [PubMed] [Google Scholar]
- 218.Jones G, Hynes KL, Dwyer T. The association between breastfeeding, maternal smoking in utero, and birth weight with bone mass and fractures in adolescents: a 16-year longitudinal study. Osteoporos Int. 2013;24:1605–1611. doi: 10.1007/s00198-012-2207-3. [DOI] [PubMed] [Google Scholar]
- 219.Ma D, Jones G. The association between bone mineral density, metacarpal morphometry, and upper limb fractures in children: a population-based case–control study. J Clin Endocrinol Metab. 2003;88:1486–1491. doi: 10.1210/jc.2002-021682. [DOI] [PubMed] [Google Scholar]
- 220.Fewtrell MS, Kennedy K, Murgatroyd PR, Williams JE, Chomtho S, Lucas A. Breast-feeding and formula feeding in healthy term infants and bone health at age 10 years. Br J Nutr. 2013;110:1061–1067. doi: 10.1017/S0007114512006149. [DOI] [PubMed] [Google Scholar]
- 221.Young RJ, Antonson DL, Ferguson PW, Murray ND, Merkel K, Moore TE. Neonatal and infant feeding: effect on bone density at 4 years. J Pediatr Gastroenterol Nutr. 2005;41:88–93. doi: 10.1097/01.mpg.0000162481.81900.e6. [DOI] [PubMed] [Google Scholar]
- 222.Bonny AE, Secic M, Cromer BA. Relationship between weight and bone mineral density in adolescents on hormonal contraception. J Pediatr Adolesc Gynecol. 2011;24:35–38. doi: 10.1016/j.jpag.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lloyd T, Taylor DS, Lin HM, Matthews AE, Eggli DF, Legro RS. Oral contraceptive use by teenage women does not affect peak bone mass: a longitudinal study. Fertil Steril. 2000;74:734–738. doi: 10.1016/s0015-0282(00)00719-6. [DOI] [PubMed] [Google Scholar]
- 224.Lloyd T, Petit MA, Lin HM, Beck TJ. Lifestyle factors and the development of bone mass and bone strength in young women. J Pediatr. 2004;144:776–782. doi: 10.1016/j.jpeds.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 225.Lara-Torre E, Edwards CP, Perlman S, Hertweck SP. Bone mineral density in adolescent females using depot medroxyprogesterone acetate. J Pediatr Adolesc Gynecol. 2004;17:17–21. doi: 10.1016/j.jpag.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 226.Cromer BA, Bonny AE, Stager M, Lazebnik R, Rome E, Ziegler J, Camlin-Shingler K, Secic M. Bone mineral density in adolescent females using injectable or oral contraceptives: a 24-month prospective study. Fertil Steril. 2008;90:2060–2067. doi: 10.1016/j.fertnstert.2007.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Rome E, Ziegler J, Secic M, Bonny A, Stager M, Lazebnik R, Cromer BA. Bone biochemical markers in adolescent girls using either depot medroxyprogesterone acetate or an oral contraceptive. J Pediatr Adolesc Gynecol. 2004;17:373–377. doi: 10.1016/j.jpag.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 228.Pikkarainen E, Lehtonen-Veromaa M, Mottonen T, Kautiainen H, Viikari J. Estrogen-progestin contraceptive use during adolescence prevents bone mass acquisition: a 4-year follow-up study. Contraception. 2008;78:226–231. doi: 10.1016/j.contraception.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 229.Biason TP, Goldberg TB, Kurokawa CS, Moretto MR, Teixeira AS, Nunes HR. Low-dose combined oral contraceptive use is associated with lower bone mineral content variation in adolescents over a 1-year period. BMC Endocr Disord. 2015;15:15. doi: 10.1186/s12902-015-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Walsh JS, Eastell R, Peel NFA. Effects of Depot medroxyprogesterone acetate on bone density and bone metabolism before and after peak bone mass: a case–control study. J Clin Endocrinol Metab. 2008;93:1317–1323. doi: 10.1210/jc.2007-2201. [DOI] [PubMed] [Google Scholar]
- 231.Korkor AB, Eastwood D, Bretzmann C. Effects of gender, alcohol, smoking, and dairy consumption on bone mass in Wisconsin adolescents. WMJ. 2009;108:181–188. [PubMed] [Google Scholar]
- 232.Dorn LD, Pabst S, Sontag LM, Kalkwarf HJ, Hillman JB, Susman EJ. Bone mass, depressive, and anxiety symptoms in adolescent girls: variation by smoking and alcohol use. J Adolesc Health. 2011;49:498–504. doi: 10.1016/j.jadohealth.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Dorn LD, Beal SJ, Kalkwarf HJ, Pabst S, Noll JG, Susman EJ. Longitudinal impact of substance use and depressive symptoms on bone accrual among girls aged 11–19 years. J Adolesc Health. 2013;52:393–399. doi: 10.1016/j.jadohealth.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Winther A, Dennison E, Ahmed LA, Furberg AS, Grimnes G, Jorde R, Gjesdal CG, Emaus N. The Tromso study: fit futures: a study of Norwegian adolescents’ lifestyle and bone health. Arch Osteoporos. 2014;9:185. doi: 10.1007/s11657-014-0185-0. [DOI] [PubMed] [Google Scholar]
- 235.Lucas R, Fraga S, Ramos E, Barros H. Early initiation of smoking and alcohol drinking as a predictor of lower forearm bone mineral density in late adolescence: a cohort study in girls. PLoS One. 2012;7:e46940. doi: 10.1371/journal.pone.0046940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kyriazopoulos P, Trovas G, Charopoulos J, Antonogiannakis E, Galanos A, Lyritis G. Lifestyle factors and forearm bone density in young Greek men. Clin Endocrinol (Oxf) 2006;65:234–238. doi: 10.1111/j.1365-2265.2006.02581.x. [DOI] [PubMed] [Google Scholar]
- 237.Elgan C, Dykes AK, Samsioe G. Bone mineral density and lifestyle among female students aged 16–24 years. Gynecol Endocrinol. 2002;16:91–98. doi: 10.1080/gye.16.2.91.98. [DOI] [PubMed] [Google Scholar]
- 238.Eleftheriou KI, Rawal JS, James LE, Payne JR, Loosemore M, Pennell DJ, World M, Drenos F, Haddad FS, Humphries SE, Sanders J, Montgomery HE. Bone structure and geometry in young men: the influence of smoking, alcohol intake and physical activity. Bone. 2013;52:17–26. doi: 10.1016/j.bone.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 239.Lorentzon M, Mellstrom D, Haug E, Ohlsson C. Smoking is associated with lower bone mineral density and reduced cortical thickness in young men. J Clin Endocrinol Metab. 2007;92:497–503. doi: 10.1210/jc.2006-1294. [DOI] [PubMed] [Google Scholar]
- 240.Elgan C, Samsioe G, Dykes AK. Influence of smoking and oral contraceptives on bone mineral density and bone remodeling in young women: a 2-year study. Contraception. 2003;67:439–447. doi: 10.1016/s0010-7824(03)00048-9. [DOI] [PubMed] [Google Scholar]
- 241.Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin d supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008;23:741–749. doi: 10.1359/jbmr.080102. [DOI] [PubMed] [Google Scholar]
- 242.Yu CC, Sung RY, So RC, Lui KC, Lau W, Lam PK, Lau EM. Effects of strength training on body composition and bone mineral content in children who are obese. J Strength Cond Res. 2005;19:667–672. doi: 10.1519/14994.1. [DOI] [PubMed] [Google Scholar]
- 243.Nichols DL, Sanborn CF, Love AM. Resistance training and bone mineral density in adolescent females. J Pediatr. 2001;139:494–500. doi: 10.1067/mpd.2001.116698. [DOI] [PubMed] [Google Scholar]
- 244.Heinonen A, Sievanen H, Kannus P, Oja P, Pasanen M, Vuori I. High-impact exercise and bones of growing girls: a 9-month controlled trial. Osteoporos Int. 2000;11:1010–1017. doi: 10.1007/s001980070021. [DOI] [PubMed] [Google Scholar]
- 245.Molgaard C, Thomsen BL, Michaelsen KF. The influence of calcium intake and physical activity on bone mineral content and bone size in healthy children and adolescents. Osteoporos Int. 2001;12:887–894. doi: 10.1007/s001980170042. [DOI] [PubMed] [Google Scholar]
- 246.Biddle SJ, Gorely T, Pearson N, Bull FC. An assessment of self-reported physical activity instruments in young people for population surveillance: Project ALPHA. Int J Behav Nutr Phys Act. 2011;8:1. doi: 10.1186/1479-5868-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Helmerhorst HJ, Brage S, Warren J, Besson H, Ekelund U. A systematic review of reliability and objective criterion-related validity of physical activity questionnaires. Int J Behav Nutr Phys Act. 2012;9:103. doi: 10.1186/1479-5868-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Janz KF, Letuchy EM, Burns TL, Eichenberger Gilmore JM, Torner JC, Levy SM. Objectively measured physical activity trajectories predict adolescent bone strength: Iowa Bone Development Study. Br J Sports Med. 2014;48:1032–1036. doi: 10.1136/bjsports-2014-093574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Specker B, Binkley T, Fahrenwald N. Increased periosteal circumference remains present 12 months after an exercise intervention in preschool children. Bone. 2004;35:1383–1388. doi: 10.1016/j.bone.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 250.Macdonald HM, Cooper DM, McKay HA. Anterior-posterior bending strength at the tibial shaft increases with physical activity in boys: evidence for non-uniform geometric adaptation. Osteoporos Int. 2009;20:61–70. doi: 10.1007/s00198-008-0636-9. [DOI] [PubMed] [Google Scholar]
- 251.Jackowski SA, Kontulainen SA, Cooper DM, Lanovaz JL, Beck TJ, Baxter-Jones AD. Adolescent physical activity and bone strength at the proximal femurin adulthood. Med Sci Sports Exerc. 2014;46:736–744. doi: 10.1249/MSS.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 252.Duckham RL, Baxter-Jones AD, Johnston JD, Vatanparast H, Cooper D, Kontulainen S. Does physical activity in adolescence have site-specific and sex-specific benefits on young adult bone size, content, and estimated strength? J Bone Miner Res. 2014;29:479–486. doi: 10.1002/jbmr.2055. [DOI] [PubMed] [Google Scholar]
- 253.Mangano KM, Sahni S, Kerstetter JE, Kenny AM, Hannan MT. Polyunsaturated fatty acids and their relation with bone and muscle health in adults. Curr Osteoporos Rep. 2013;11:203–212. doi: 10.1007/s11914-013-0149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Damsgaard CT, Molgaard C, Matthiessen J, Gyldenlove SN, Lauritzen L. The effects of n-3 long-chain polyunsaturated fatty acids on bone formation and growth factors in adolescent boys. Pediatr Res. 2012;71:713–719. doi: 10.1038/pr.2012.28. [DOI] [PubMed] [Google Scholar]
- 255.Weaver CM, Proulx WR, Heaney R. Choices for achieving adequate dietary calcium with a vegetarian diet. Am J Clin Nutr. 1999;70:543S–548S. doi: 10.1093/ajcn/70.3.543s. [DOI] [PubMed] [Google Scholar]
- 256.Thacher TD, Fischer PR, Strand MA, Pettifor JM. Nutritional rickets around the world: causes and future directions. Ann Trop Paediatr. 2006;26:1–16. doi: 10.1179/146532806X90556. [DOI] [PubMed] [Google Scholar]
- 257.Pettifor JM. Nutritional rickets: deficiency of vitamin D, calcium, or both? Am J Clin Nutr. 2004;80:1725S–1729S. doi: 10.1093/ajcn/80.6.1725S. [DOI] [PubMed] [Google Scholar]
- 258.Braun M, Palacios C, Wigertz K, Jackman LA, Bryant RJ, McCabe LD, Martin BR, McCabe GP, Peacock M, Weaver CM. Racial differences in skeletal calcium retention in adolescent girls with varied controlled calcium intakes. Am J Clin Nutr. 2007;85:1657–1663. doi: 10.1093/ajcn/85.6.1657. [DOI] [PubMed] [Google Scholar]
- 259.Jackman LA, Millane SS, Martin BR, Wood OB, McCabe GP, Peacock M, Weaver CM. Calcium retention in relation to calcium intake and postmenarcheal age in adolescent females. Am J Clin Nutr. 1997;66:327–333. doi: 10.1093/ajcn/66.2.327. [DOI] [PubMed] [Google Scholar]
- 260.Wu L, Martin BR, Braun MM, Wastney ME, McCabe GP, McCabe LD, DiMeglio LA, Peacock M, Weaver CM. Calcium requirements and metabolism in Chinese-American boys and girls. J Bone Miner Res. 2010;25:1842–1849. doi: 10.1002/jbmr.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Braun M, Martin BR, Kern M, McCabe GP, Peacock M, Jiang Z, Weaver CM. Calcium retention in adolescent boys on a range of controlled calcium intakes. Am J Clin Nutr. 2006;84:414–418. doi: 10.1093/ajcn/84.1.414. [DOI] [PubMed] [Google Scholar]
- 262.Palacios C, Martin BR, McCabe GP, McCabe L, Peacock M, Weaver CM. Dietary calcium requirements do not differ between Mexican-American boys and girls. J Nutr. 2014;144:1167–1173. doi: 10.3945/jn.113.188318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Del Valle HB, Yaktine AL, Taylor CL, Ross AC (2011) Dietary reference intakes for calcium and vitamin D. National Academies Press [PubMed]
- 264.Looker AC (2006) Dietary calcium: recommendations and intakes around the world. In: Calcium in human health. Humana Press, Totowa, pp 105–127
- 265.Heaney RP. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr Rev. 2014;72:48–54. doi: 10.1111/nure.12090. [DOI] [PubMed] [Google Scholar]
- 266.Winzenberg T, Powell S, Shaw KA, Jones G. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ. 2011;342:c7254. doi: 10.1136/bmj.c7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.DeLuca HF, Sicinski RR, Tanaka Y, Stern PH, Smith CM. Biological activity of 1,25-dihydroxyvitamin D2 and 24-epi-1,25-dihydroxyvitamin D2. Am J Physiol. 1988;254:E402–E406. doi: 10.1152/ajpendo.1988.254.4.E402. [DOI] [PubMed] [Google Scholar]
- 268.Lewis RD, Laing EM, Hill Gallant KM, Hall DB, McCabe GP, Hausman DB, Martin BR, Warden SJ, Peacock M, Weaver CM. A randomized trial of vitamin D(3) supplementation in children: dose–response effects on vitamin D metabolites and calcium absorption. J Clin Endocrinol Metab. 2013;98:4816–4825. doi: 10.1210/jc.2013-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Wigertz K, Palacios C, Jackman LA, Martin BR, McCabe LD, McCabe GP, Peacock M, Pratt JH, Weaver CM. Racial differences in calcium retention in response to dietary salt in adolescent girls. Am J Clin Nutr. 2005;81:845–850. doi: 10.1093/ajcn/81.4.845. [DOI] [PubMed] [Google Scholar]
- 270.Palacios C, Wigertz K, Braun M, Martin BR, McCabe GP, McCabe L, Pratt JH, Peacock M, Weaver CM. Magnesium retention from metabolic-balance studies in female adolescents: impact of race, dietary salt, and calcium. Am J Clin Nutr. 2013;97:1014–1019. doi: 10.3945/ajcn.112.039867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Palacios C, Wigertz K, Martin BR, Braun M, Pratt JH, Peacock M, Weaver CM. Racial differences in potassium homeostasis in response to differences in dietary sodium in girls. Am J Clin Nutr. 2010;91:597–603. doi: 10.3945/ajcn.2009.28400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Szulc P, Delmas P (1994) Is there a role for vitamin K deficiency in osteoporosis? In: Challenges of modern medicine, nutritional aspects of osteoporosis (Proceedings of the Second International Symposium on Osteoporosis, Lausanne). Serono Symposia Publications, Rome, pp 357–366
- 273.National Research Council . Fluoride in drinking water: a scientific review of EPA’s standards. Washington: The National Academies Press; 2006. [Google Scholar]
- 274.Bratteb LE, Samuelson G, Sandhagen B, Mallmin H, Lantz H, Sjostrom L. Whole-body mineral measurements in Swedish adolescents at 17 years compared to 15 years of age. Acta Paediatr. 2002;91:1031–1038. doi: 10.1080/080352502760311502. [DOI] [PubMed] [Google Scholar]
- 275.US Department of Agriculture . Dietary guidelines for Americans, 2010. 7. Washington: US Government Printing Office; 2010. [Google Scholar]
- 276.Weaver CM. Potassium and health. Adv Nutr. 2013;4:368S–377S. doi: 10.3945/an.112.003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Weaver CM, Alekel DL, Ward WE, Ronis MJ. Flavonoid intake and bone health. J Nutr Gerontol Geriatr. 2012;31:239–253. doi: 10.1080/21551197.2012.698220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kristensen M, Jensen M, Kudsk J, Henriksen M, Molgaard C. Short-term effects on bone turnover of replacing milk with cola beverages: a 10-day interventional study in young men. Osteoporos Int. 2005;16:1803–1808. doi: 10.1007/s00198-005-1935-z. [DOI] [PubMed] [Google Scholar]
- 279.Wastney ME, Martin BR, Peacock M, Smith D, Jiang XY, Jackman LA, Weaver CM. Changes in calcium kinetics in adolescent girls induced by high calcium intake. J Clin Endocrinol Metab. 2000;85:4470–4475. doi: 10.1210/jcem.85.12.7004. [DOI] [PubMed] [Google Scholar]
- 280.Sjogren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, Backhed F, Ohlsson C. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27:1357–1367. doi: 10.1002/jbmr.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Abrams SA, Griffin IJ, Hawthorne KM. Young adolescents who respond to an inulin-type fructan substantially increase total absorbed calcium and daily calcium accretion to the skeleton. J Nutr. 2007;137:2524S–2526S. doi: 10.1093/jn/137.11.2524S. [DOI] [PubMed] [Google Scholar]
- 282.Whisner CM, Martin BR, Nakatsu CH, McCabe GP, McCabe LD, Peacock M, Weaver CM. Soluble maize fibre affects short-term calcium absorption in adolescent boys and girls: a randomised controlled trial using dual stable isotopic tracers. Br J Nutr. 2014;112:446–456. doi: 10.1017/S0007114514000981. [DOI] [PubMed] [Google Scholar]
- 283.Whisner CM, Martin BR, Schoterman MH, Nakatsu CH, McCabe LD, McCabe GP, Wastney ME, van den Heuvel EG, Weaver CM. Galacto-oligosaccharides increase calcium absorption and gut bifidobacteria in young girls: a double-blind cross-over trial. Br J Nutr. 2013;110:1292–1303. doi: 10.1017/S000711451300055X. [DOI] [PubMed] [Google Scholar]
- 284.Specker BL, Beck A, Kalkwarf H, Ho M. Randomized trial of varying mineral intake on total body bone mineral accretion during the first year of life. Pediatrics. 1997;99:E12. doi: 10.1542/peds.99.6.e12. [DOI] [PubMed] [Google Scholar]
- 285.Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 286.Polatti F, Perotti F, Filippa N, Gallina D, Nappi RE. Bone mass and long-term monophasic oral contraceptive treatment in young women. Contraception. 1995;51:221–224. doi: 10.1016/0010-7824(95)00036-a. [DOI] [PubMed] [Google Scholar]
- 287.Warholm L, Petersen KR, Ravn P. Combined oral contraceptives’ influence on weight, body composition, height, and bone mineral density in girls younger than 18 years: a systematic review. Eur J Contracept Reprod Health Care. 2012;17:245–253. doi: 10.3109/13625187.2012.692411. [DOI] [PubMed] [Google Scholar]
- 288.Tremollieres F. Impact of oral contraceptive on bone metabolism. Best Pract Res Clin Endocrinol Metab. 2013;27:47–53. doi: 10.1016/j.beem.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 289.Ziglar S, Hunter TS. The effect of hormonal oral contraception on acquisition of peak bone mineral density of adolescents and young women. J Pharm Pract. 2012;25:331–340. doi: 10.1177/0897190012442066. [DOI] [PubMed] [Google Scholar]
- 290.Harel Z, Johnson CC, Gold MA, Cromer B, Peterson E, Burkman R, Stager M, Brown R, Bruner A, Coupey S, Hertweck P, Bone H, Wolter K, Nelson A, Marshall S, Bachrach LK. Recovery of bone mineral density in adolescents following the use of depot medroxyprogesterone acetate contraceptive injections. Contraception. 2010;81:281–291. doi: 10.1016/j.contraception.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 291.Wosje KS, Kalkwarf HJ. Bone density in relation to alcohol intake among men and women in the United States. Osteoporos Int. 2007;18:391–400. doi: 10.1007/s00198-006-0249-0. [DOI] [PubMed] [Google Scholar]
- 292.Office of Juvenile Justice and Delinquency Prevention . Drinking in America: myths, reality and prevention policy. Washington: US Department of Justice, Office of Justice Programs, Office of Juvenile Justice and Delinquency Prevention; 2005. [Google Scholar]
- 293.US Centers for Disease Control and Prevention (2013) Trends in current cigarette smoking. http://www.cdc.gov/tobacco/data_statistics/tables/trends/cig_smoking/index.htm. Accessed 5 Aug 2014
- 294.Blum M, Harris SS, Must A, Phillips SM, Rand WM, Dawson-Hughes B. Household tobacco smoke exposure is negatively associated with premenopausal bone mass. Osteoporos Int. 2002;13:663–668. doi: 10.1007/s001980200090. [DOI] [PubMed] [Google Scholar]
- 295.MacKelvie KJ, Petit MA, Khan KM, Beck TJ, McKay HA. Bone mass and structure are enhanced following a 2-year randomized controlled trial of exercise in prepubertal boys. Bone. 2004;34:755–764. doi: 10.1016/j.bone.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 296.Weeks BK, Young CM, Beck BR. Eight months of regular in-school jumping improves indices of bone strength in adolescent boys and Girls: the POWER PE study. J Bone Miner Res. 2008;23:1002–1011. doi: 10.1359/jbmr.080226. [DOI] [PubMed] [Google Scholar]
- 297.McKay HA, MacLean L, Petit M, MacKelvie-O’Brien K, Janssen P, Beck T, Khan KM. “Bounce at the Bell”: a novel program of short bouts of exercise improves proximal femur bone mass in early pubertal children. Br J Sports Med. 2005;39:521–526. doi: 10.1136/bjsm.2004.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Linden C, Alwis G, Ahlborg H, Gardsell P, Valdimarsson O, Stenevi-Lundgren S, Besjakov J, Karlsson MK. Exercise, bone mass and bone size in prepubertal boys: one-year data from the pediatric osteoporosis prevention study. Scand J Med Sci Sports. 2007;17:340–347. doi: 10.1111/j.1600-0838.2006.00568.x. [DOI] [PubMed] [Google Scholar]
- 299.Macdonald HM, Kontulainen SA, Petit MA, Beck TJ, Khan KM, McKay HA. Does a novel school-based physical activity model benefit femoral neck bone strength in pre- and early pubertal children? Osteoporos Int. 2008;19:1445–1456. doi: 10.1007/s00198-008-0589-z. [DOI] [PubMed] [Google Scholar]
- 300.Alwis G, Linden C, Stenevi-Lundgren S, Ahlborg HG, Besjakov J, Gardsell P, Karlsson MK. A one-year exercise intervention program in pre-pubertal girls does not influence hip structure. BMC Musculoskelet Disord. 2008;9:9. doi: 10.1186/1471-2474-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Greene DA, Wiebe PN, Naughton GA. Influence of drop-landing exercises on bone geometry and biomechanical properties in prepubertal girls: a randomized controlled study. Calcif Tissue Int. 2009;85:94–103. doi: 10.1007/s00223-009-9253-7. [DOI] [PubMed] [Google Scholar]
- 302.Anliker E, Dick C, Rawer R, Toigo M. Effects of jumping exercise on maximum ground reaction force and bone in 8- to 12-year-old boys and girls: a 9-month randomized controlled trial. J Musculoskelet Neuronal Interact. 2012;12:56–67. [PubMed] [Google Scholar]
- 303.Detter F, Rosengren BE, Dencker M, Lorentzon M, Nilsson JA, Karlsson MK. A 6-year exercise program improves skeletal traits without affecting fracture risk: a prospective controlled study in 2621 children. J Bone Miner Res. 2014;29:1325–1336. doi: 10.1002/jbmr.2168. [DOI] [PubMed] [Google Scholar]
- 304.Metcalf B, Henley W, Wilkin T. Effectiveness of intervention on physical activity of children: systematic review and meta-analysis of controlled trials with objectively measured outcomes (EarlyBird 54) BMJ. 2012;345:e5888. doi: 10.1136/bmj.e5888. [DOI] [PubMed] [Google Scholar]
- 305.Deere K, Sayers A, Rittweger J, Tobias JH. Habitual levels of high, but not moderate or low, impact activity are positively related to hip BMD and geometry: results from a population-based study of adolescents. J Bone Miner Res. 2012;27:1887–1895. doi: 10.1002/jbmr.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Fuchs RK, Bauer JJ, Snow CM. Jumping improves hip and lumbar spine bone mass in prepubescent children: a randomized controlled trial. J Bone Miner Res. 2001;16:148–156. doi: 10.1359/jbmr.2001.16.1.148. [DOI] [PubMed] [Google Scholar]
- 307.Hind K, Burrows M. Weight-bearing exercise and bone mineral accrual in children and adolescents: a review of controlled trials. Bone. 2007;40:14–27. doi: 10.1016/j.bone.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 308.Ju YI, Sone T, Ohnaru K, Tanaka K, Yamaguchi H, Fukunaga M. Effects of different types of jump impact on trabecular bone mass and microarchitecture in growing rats. PLoS One. 2014;9:e107953. doi: 10.1371/journal.pone.0107953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Office of the Surgeon General . Bone health and osteoporosis: a report of the Surgeon General. Rockville: US Department of Health and Human Services, Office of the Surgeon General; 2004. [PubMed] [Google Scholar]
- 310.Umemura Y, Ishiko T, Tsujimoto H, Miura H, Mokushi N, Suzuki H. Effects of jump training on bone hypertrophy in young and old rats. Int J Sports Med. 1995;16:364–367. doi: 10.1055/s-2007-973021. [DOI] [PubMed] [Google Scholar]
- 311.Hing KA. Bone repair in the twenty-first century: biology, chemistry or engineering? Philos Transact A Math Phys Eng Sci. 2004;362:2821–2850. doi: 10.1098/rsta.2004.1466. [DOI] [PubMed] [Google Scholar]
- 312.Westbroek I, van der Plas A, de Rooij KE, Klein-Nulend J, Nijweide PJ. Expression of serotonin receptors in bone. J Biol Chem. 2001;276:28961–28968. doi: 10.1074/jbc.M101824200. [DOI] [PubMed] [Google Scholar]
- 313.Stanford CM, Brand RA. Toward an understanding of implant occlusion and strain adaptive bone modeling and remodeling. J Prosthet Dent. 1999;81:553–561. doi: 10.1016/s0022-3913(99)70209-x. [DOI] [PubMed] [Google Scholar]
- 314.Bachrach LK, Guido D, Katzman D, Litt IF, Marcus R. Decreased bone density in adolescent girls with anorexia nervosa. Pediatrics. 1990;86:440–447. [PubMed] [Google Scholar]
- 315.Soyka LA, Grinspoon S, Levitsky LL, Herzog DB, Klibanski A. The effects of anorexia nervosa on bone metabolism in female adolescents. J Clin Endocrinol Metab. 1999;84:4489–4496. doi: 10.1210/jcem.84.12.6207. [DOI] [PubMed] [Google Scholar]
- 316.Soyka LA, Misra M, Frenchman A, Miller KK, Grinspoon S, Schoenfeld DA, Klibanski A. Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2002;87:4177–4185. doi: 10.1210/jc.2001-011889. [DOI] [PubMed] [Google Scholar]
- 317.Grinspoon S, Thomas E, Pitts S, Gross E, Mickley D, Miller K, Herzog D, Klibanski A. Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Ann Intern Med. 2000;133:790–794. doi: 10.7326/0003-4819-133-10-200011210-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Putman MS, Milliren CE, Derrico N, Uluer A, Sicilian L, Lapey A, Sawicki G, Gordon CM, Bouxsein ML, Finkelstein JS. Compromised bone microarchitecture and estimated bone strength in young adults with cystic fibrosis. J Clin Endocrinol Metab. 2014;99:3399–3407. doi: 10.1210/jc.2014-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Wu FJ, Sheu SY, Lin HC. Osteoporosis is associated with antiepileptic drugs: a population-based study. Epileptic Disord. 2014;16:333–342. doi: 10.1684/epd.2014.0673. [DOI] [PubMed] [Google Scholar]
- 320.Krebs-Smith SM, Guenther PM, Subar AF, Kirkpatrick SI, Dodd KW. Americans do not meet federal dietary recommendations. J Nutr. 2010;140:1832–1838. doi: 10.3945/jn.110.124826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Fulgoni VL, 3rd, Keast DR, Bailey RL, Dwyer J. Foods, fortificants, and supplements: where do Americans get their nutrients? J Nutr. 2011;141:1847–1854. doi: 10.3945/jn.111.142257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Mattila PH, Piironen VI, Uusi-Rauva EJ, Koivistoinen PE. Vitamin D contents in edible mushrooms. J Agric Food Chem. 1994;42:2449–2453. [Google Scholar]
- 323.Calvo MS, Whiting SJ, Barton CN. Vitamin D fortification in the United States and Canada: current status and data needs. Am J Clin Nutr. 2004;80:1710S–1716S. doi: 10.1093/ajcn/80.6.1710S. [DOI] [PubMed] [Google Scholar]
- 324.Hohman EE, Martin BR, Lachcik PJ, Gordon DT, Fleet JC, Weaver CM. Bioavailability and efficacy of vitamin D2 from UV-irradiated yeast in growing, vitamin D-deficient rats. J Agric Food Chem. 2011;59:2341–2346. doi: 10.1021/jf104679c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Taylor CL, Patterson KY, Roseland JM, Wise SA, Merkel JM, Pehrsson PR, Yetley EA. Including food 25-hydroxyvitamin D in intake estimates may reduce the discrepancy between dietary and serum measures of vitamin D status. J Nutr. 2014;144:654–659. doi: 10.3945/jn.113.189811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.US Department of Health and Human Services . Physical activity guidelines for Americans. Washington: US Government Printing Office; 2008. [Google Scholar]
- 327.US Institute of Medicine . Educating the student body: taking physical activity and physical education to school. Washington: National Academies Press; 2013. [PubMed] [Google Scholar]
- 328.Eaton DK, Kann L, Kinchen S, Shanklin S, Flint KH, Hawkins J, Harris WA, Lowry R, McManus T, Chyen D, Whittle L, Lim C, Wechsler H. Youth risk behavior surveillance—United States, 2011. MMWR Surveill Summ. 2012;61:1–162. [PubMed] [Google Scholar]
- 329.Physical Activity Guidelines for Americans Midcourse Report Subcommittee of the President’s Council on Fitness Sports & Nutrition . Physical activity guidelines for Americans midcourse report: strategies to increase physical activity among youth. Washington: US Department of Health and Human Services; 2012. [Google Scholar]
- 330.Rozen GS, Rennert G, Dodiuk-Gad RP, Rennert HS, Ish-Shalom N, Diab G, Raz B, Ish-Shalom S. Calcium supplementation provides an extended window of opportunity for bone mass accretion after menarche. Am J Clin Nutr. 2003;78:993–998. doi: 10.1093/ajcn/78.5.993. [DOI] [PubMed] [Google Scholar]
- 331.Dodiuk-Gad RP, Rozen GS, Rennert G, Rennert HS, Ish-Shalom S. Sustained effect of short-term calcium supplementation on bone mass in adolescent girls with low calcium intake. Am J Clin Nutr. 2005;81:168–174. doi: 10.1093/ajcn/81.1.168. [DOI] [PubMed] [Google Scholar]
- 332.Molgaard C, Thomsen BL, Michaelsen KF. Effect of habitual dietary calcium intake on calcium supplementation in 12–14-y-old girls. Am J Clin Nutr. 2004;80:1422–1427. doi: 10.1093/ajcn/80.5.1422. [DOI] [PubMed] [Google Scholar]
- 333.Matkovic V, Goel PK, Badenhop-Stevens NE, Landoll JD, Li B, Ilich JZ, Skugor M, Nagode LA, Mobley SL, Ha EJ, Hangartner TN, Clairmont A. Calcium supplementation and bone mineral density in females from childhood to young adulthood: a randomized controlled trial. Am J Clin Nutr. 2005;81:175–188. doi: 10.1093/ajcn/81.1.175. [DOI] [PubMed] [Google Scholar]
- 334.Khadilkar A, Kadam N, Chiplonkar S, Fischer PR, Khadilkar V. School-based calcium-vitamin D with micronutrient supplementation enhances bone mass in underprivileged Indian premenarchal girls. Bone. 2012;51:1–7. doi: 10.1016/j.bone.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 335.Wosje KS, Khoury PR, Claytor RP, Copeland KA, Hornung RW, Daniels SR, Kalkwarf HJ. Dietary patterns associated with fat and bone mass in young children. Am J Clin Nutr. 2010;92:294–303. doi: 10.3945/ajcn.2009.28925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Afghani A, Xie B, Wiswell RA, Gong J, Li Y, Anderson Johnson C. Bone mass of Asian adolescents in China: influence of physical activity and smoking. Med Sci Sports Exerc. 2003;35:720–729. doi: 10.1249/01.MSS.0000064940.76574.BD. [DOI] [PubMed] [Google Scholar]
- 337.Witzke KA, Snow CM. Effects of plyometric jump training on bone mass in adolescent girls. Med Sci Sports Exerc. 2000;32:1051–1057. doi: 10.1097/00005768-200006000-00003. [DOI] [PubMed] [Google Scholar]
- 338.McKay HA, Petit MA, Schutz RW, Prior JC, Barr SI, Khan KM. Augmented trochanteric bone mineral density after modified physical education classes: a randomized school-based exercise intervention study in prepubescent and early pubescent children. J Pediatr. 2000;136:156–162. doi: 10.1016/s0022-3476(00)70095-3. [DOI] [PubMed] [Google Scholar]
- 339.Mackelvie KJ, McKay HA, Petit MA, Moran O, Khan KM. Bone mineral response to a 7-month randomized controlled, school-based jumping intervention in 121 prepubertal boys: associations with ethnicity and body mass index. J Bone Miner Res. 2002;17:834–844. doi: 10.1359/jbmr.2002.17.5.834. [DOI] [PubMed] [Google Scholar]
- 340.Laing EM, Massoni JA, Nickols-Richardson SM, Modlesky CM, O’Connor PJ, Lewis RD. A prospective study of bone mass and body composition in female adolescent gymnasts. J Pediatr. 2002;141:211–216. doi: 10.1067/mpd.2002.126599. [DOI] [PubMed] [Google Scholar]
- 341.MacKelvie KJ, Khan KM, Petit MA, Janssen PA, McKay HA. A school-based exercise intervention elicits substantial bone health benefits: a 2-year randomized controlled trial in girls. Pediatrics. 2003;112:e447. doi: 10.1542/peds.112.6.e447. [DOI] [PubMed] [Google Scholar]
- 342.Van Langendonck L, Claessens AL, Vlietinck R, Derom C, Beunen G. Influence of weight-bearing exercises on bone acquisition in prepubertal monozygotic female twins: a randomized controlled prospective study. Calcif Tissue Int. 2003;72:666–674. doi: 10.1007/s00223-002-2030-5. [DOI] [PubMed] [Google Scholar]
- 343.Laing EM, Wilson AR, Modlesky CM, O’Connor PJ, Hall DB, Lewis RD. Initial years of recreational artistic gymnastics training improves lumbar spine bone mineral accrual in 4- to 8-year-old females. J Bone Miner Res. 2005;20:509–519. doi: 10.1359/JBMR.041127. [DOI] [PubMed] [Google Scholar]
- 344.Valdimarsson O, Linden C, Johnell O, Gardsell P, Karlsson MK. Daily physical education in the school curriculum in prepubertal girls during 1 year is followed by an increase in bone mineral accrual and bone width—data from the prospective controlled Malmo pediatric osteoporosis prevention study. Calcif Tissue Int. 2006;78:65–71. doi: 10.1007/s00223-005-0096-6. [DOI] [PubMed] [Google Scholar]
- 345.Linden C, Ahlborg HG, Besjakov J, Gardsell P, Karlsson MK. A school curriculum-based exercise program increases bone mineral accrual and bone size in prepubertal girls: two-year data from the Pediatric Osteoporosis Prevention (POP) study. J Bone Miner Res. 2006;21:829–835. doi: 10.1359/jbmr.060304. [DOI] [PubMed] [Google Scholar]
- 346.Barbeau P, Johnson MH, Howe CA, Allison J, Davis CL, Gutin B, Lemmon CR. Ten months of exercise improves general and visceral adiposity, bone, and fitness in black girls. Obesity (Silver Spring) 2007;15:2077–2085. doi: 10.1038/oby.2007.247. [DOI] [PubMed] [Google Scholar]
- 347.Schneider M, Dunton GF, Bassin S, Graham DJ, Eliakim AF, Cooper DM. Impact of a school-based physical activity intervention on fitness and bone in adolescent females. J Phys Act Health. 2007;4:17–29. doi: 10.1123/jpah.4.1.17. [DOI] [PubMed] [Google Scholar]
- 348.Meyer U, Romann M, Zahner L, Schindler C, Puder JJ, Kraenzlin M, Rizzoli R, Kriemler S. Effect of a general school-based physical activity intervention on bone mineral content and density: a cluster-randomized controlled trial. Bone. 2011;48:792–797. doi: 10.1016/j.bone.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 349.Silva CC, Goldberg TB, Teixeira AS, Dalmas JC. The impact of different types of physical activity on total and regional bone mineral density in young Brazilian athletes. J Sports Sci. 2011;29:227–234. doi: 10.1080/02640414.2010.529456. [DOI] [PubMed] [Google Scholar]
- 350.Lehtonen-Veromaa M, Mottonen T, Irjala K, Nuotio I, Leino A, Viikari J. A 1-year prospective study on the relationship between physical activity, markers of bone metabolism, and bone acquisition in peripubertal girls. J Clin Endocrinol Metab. 2000;85:3726–3732. doi: 10.1210/jcem.85.10.6889. [DOI] [PubMed] [Google Scholar]
- 351.Gustavsson A, Olsson T, Nordstrom P. Rapid loss of bone mineral density of the femoral neck after cessation of ice hockey training: a 6-year longitudinal study in males. J Bone Miner Res. 2003;18:1964–1969. doi: 10.1359/jbmr.2003.18.11.1964. [DOI] [PubMed] [Google Scholar]
- 352.Nurmi-Lawton JA, Baxter-Jones AD, Mirwald RL, Bishop JA, Taylor P, Cooper C, New SA. Evidence of sustained skeletal benefits from impact-loading exercise in young females: a 3-year longitudinal study. J Bone Miner Res. 2004;19:314–322. doi: 10.1359/JBMR.0301222. [DOI] [PubMed] [Google Scholar]
- 353.Janz KF, Gilmore JM, Burns TL, Levy SM, Torner JC, Willing MC, Marshall TA. Physical activity augments bone mineral accrual in young children: the Iowa Bone Development study. J Pediatr. 2006;148:793–799. doi: 10.1016/j.jpeds.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 354.Nordstrom A, Olsson T, Nordstrom P. Sustained benefits from previous physical activity on bone mineral density in males. J Clin Endocrinol Metab. 2006;91:2600–2604. doi: 10.1210/jc.2006-0151. [DOI] [PubMed] [Google Scholar]
- 355.Janz KF, Letuchy EM, Eichenberger Gilmore JM, Burns TL, Torner JC, Willing MC, Levy SM. Early physical activity provides sustained bone health benefits later in childhood. Med Sci Sports Exerc. 2010;42:1072–1078. doi: 10.1249/MSS.0b013e3181c619b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Tervo T, Nordstrom P, Nordstrom A. Effects of badminton and ice hockey on bone mass in young males: a 12-year follow-up. Bone. 2010;47:666–672. doi: 10.1016/j.bone.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 357.Erlandson MC, Kontulainen SA, Chilibeck PD, Arnold CM, Baxter-Jones AD. Bone mineral accrual in 4- to 10-year-old precompetitive, recreational gymnasts: a 4-year longitudinal study. J Bone Miner Res. 2011;26:1313–1320. doi: 10.1002/jbmr.338. [DOI] [PubMed] [Google Scholar]
- 358.Scerpella TA, Dowthwaite JN, Rosenbaum PF. Sustained skeletal benefit from childhood mechanical loading. Osteoporos Int. 2011;22:2205–2210. doi: 10.1007/s00198-010-1373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Erlandson MC, Kontulainen SA, Chilibeck PD, Arnold CM, Faulkner RA, Baxter-Jones AD. Higher premenarcheal bone mass in elite gymnasts is maintained into young adulthood after long-term retirement from sport: a 14-year follow-up. J Bone Miner Res. 2012;27:104–110. doi: 10.1002/jbmr.514. [DOI] [PubMed] [Google Scholar]
- 360.Farr JN, Laddu DR, Blew RM, Lee VR, Going SB. Effects of physical activity and muscle quality on bone development in girls. Med Sci Sports Exerc. 2013;45:2332–2340. doi: 10.1249/MSS.0b013e31829c32fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Francis SL, Letuchy EM, Levy SM, Janz KF. Sustained effects of physical activity on bone health: Iowa Bone Development Study. Bone. 2014;63:95–100. doi: 10.1016/j.bone.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Cardadeiro G, Baptista F, Rosati N, Zymbal V, Janz KF, Sardinha LB. Influence of physical activity and skeleton geometry on bone mass at the proximal femur in 10- to 12-year-old children-a longitudinal study. Osteoporos Int. 2014;25:2035–2045. doi: 10.1007/s00198-014-2729-y. [DOI] [PubMed] [Google Scholar]
- 363.Forwood MR, Baxter-Jones AD, Beck TJ, Mirwald RL, Howard A, Bailey DA. Physical activity and strength of the femoral neck during the adolescent growth spurt: a longitudinal analysis. Bone. 2006;38:576–583. doi: 10.1016/j.bone.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 364.Janz KF, Gilmore JM, Levy SM, Letuchy EM, Burns TL, Beck TJ. Physical activity and femoral neck bone strength during childhood: the Iowa Bone Development Study. Bone. 2007;41:216–222. doi: 10.1016/j.bone.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Gruodyte-Raciene R, Erlandson MC, Jackowski SA, Baxter-Jones AD. Structural strength development at the proximal femur in 4- to 10-year-old precompetitive gymnasts: a 4-year longitudinal hip structural analysis study. J Bone Miner Res. 2013;28:2592–2600. doi: 10.1002/jbmr.1986. [DOI] [PubMed] [Google Scholar]
- 366.Fulgoni VL., 3rd Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003–2004. Am J Clin Nutr. 2008;87:1554s–1557s. doi: 10.1093/ajcn/87.5.1554S. [DOI] [PubMed] [Google Scholar]