Abstract
Study Objectives:
To examine nonrapid eye movement (NREM) sleep in insomnia using high-density electroencephalography (EEG).
Methods:
All-night sleep recordings with 256 channel high-density EEG were analyzed for 8 insomnia subjects (5 females) and 8 sex and age-matched controls without sleep complaints. Spectral analyses were conducted using unpaired t-tests and topographical differences between groups were assessed using statistical non-parametric mapping. Five minute segments of deep NREM sleep were further analyzed using sLORETA cortical source imaging.
Results:
The initial topographic analysis of all-night NREM sleep EEG revealed that insomnia subjects had more high-frequency EEG activity (> 16 Hz) compared to good sleeping controls and that the difference between groups was widespread across the scalp. In addition, the analysis also showed that there was a more circumscribed difference in theta (4–8 Hz) and alpha (8–12 Hz) power bands between groups. When deep NREM sleep (N3) was examined separately, the high-frequency difference between groups diminished, whereas the higher regional alpha activity in insomnia subjects persisted. Source imaging analysis demonstrated that sensory and sensorimotor cortical areas consistently exhibited elevated levels of alpha activity during deep NREM sleep in insomnia subjects relative to good sleeping controls.
Conclusions:
These results suggest that even during the deepest stage of sleep, sensory and sensorimotor areas in insomnia subjects may still be relatively active compared to control subjects and to the rest of the sleeping brain.
Citation:
Riedner BA, Goldstein MR, Plante DT, Rumble ME, Ferrarelli F, Tononi G, Benca RM. Regional patterns of elevated alpha and high-frequency electroencephalographic activity during nonrapid eye movement sleep in chronic insomnia: a pilot study. SLEEP 2016;39(4):801–812.
Keywords: alpha-delta sleep, high-density EEG, local sleep, neuroimaging, source modeling
Significance.
Prior work has suggested that people with insomnia have difficulty with hyperarousal. Recent data also demonstrate consistently that the brain does not sleep uniformly. This work shows for the first time that even though the brain is showing widespread electroencephalographic signs characteristic of deep sleep in insomnia subjects, parts of sensory cortex are also showing rhythms that suggest these brain areas might still be awake, or at least not totally asleep, relative to controls. This finding lends support to the notion of local sleep dysregulation in insomnia.
INTRODUCTION
Insomnia is a prevalent health concern, with approximately one-third of the general population experiencing insomnia symptoms and nearly 10% meeting criteria for insomnia disorder,1,2 characterized by difficulties with sleep initiation, maintenance, or nonrestorative sleep, and accompanied by significant impairments to daytime functioning.3 Given the numerous health problems, decreased quality of life, and detriments to work performance associated with insomnia,4–8 continued investigation of the pathophysiological mechanisms and their implications for treatment is critical.
Hyperarousal, an inappropriate activation of the central and peripheral nervous system that interferes with the initiation and maintenance of sleep, is thought to be a key component of chronic insomnia.9,10 Supporting evidence for the role of hyperarousal in insomnia comes from multiple research domains, which have demonstrated heightened sympathetic combined with decreased parasympathetic activity,11,12 endocrine changes,13 as well as elevated body and brain metabolism.14–16 Notably, a large body of insomnia research has focused on the increase of high-frequency electroencephalographic activity (> 16 Hz) during sleep, especially around sleep onset, to support the idea of physiological hyperarousal.17–28 The fundamental role of electroencephalography (EEG) analysis is even further emphasized by the neurocognitive interpretation of hyperarousal, which suggests that the increase of high-frequency EEG activity reflects an important perpetuating factor in insomnia as a sign of conditioned cortical arousal that is accompanied by enhanced sensory and information processing along with increased memory formation.29
However, despite the large number of studies that have analyzed electroencephalographic power in primary insomnia, few have explored its topographic distribution. It is now relatively well established that sleep itself is not a global phenomenon.30–34 Building on the concept of local sleep, an alternative model of insomnia has been proposed that suggests that insomnia is characterized by the simultaneous occurrence of wake and sleep states in specific brain regions.35 High-density EEG (hdEEG) makes it possible to characterize regional patterns of activity during sleep and may therefore provide novel insights into the pathophysiologic mechanisms of insomnia.
An additional advantage of hdEEG is the ability to estimate cortical sources of EEG activity observed at the scalp level.36 Although neuroimaging promises to provide valuable information on the cortical and subcortical generators contributing to insomnia pathophysiology (for an excellent review of the neuroimaging studies in insomnia, see Spiegelhalder et al.37), many of the techniques utilized (e.g., magnetic resonance imaging, spectrography, and positron emission topography) are not ideally suited for studying all aspects of this disorder either because the environmental conditions are not conducive to recording during sleep or because the sampling resolution for imaging is not sufficient to capture the momentary fluctuations of sleep state and arousal level. By contrast, with hdEEG, brain activity can be recorded with millisecond resolution while allowing subjects to sleep in a much more natural environment and still achieve reasonable spatial specificity, mitigating the potential for sleep disruption and enabling a unique contribution to the brain imaging literature on insomnia. Moreover, advances in hdEEG electrode stability have made high-quality EEG data achievable for greater than 10 h, thereby offering the considerable advantage of performing longer EEG studies and significantly expanding the window for imaging during sleep.
We therefore examined nonrapid eye movement (NREM) sleep hdEEG in a group of individuals with chronic insomnia complaints and good sleeper controls with the goal of elucidating functional areas in insomnia sleep pathophysiology.
METHODS
Participant Recruitment and Eligibility
Chronic Insomnia
Insomnia participants (n = 8) were recruited through clinic referrals from Wisconsin Sleep and the Department of Psychiatry at the University of Wisconsin-Madison as part of a pilot study using high-density EEG to investigate localized patterns of activity in sleep and wakefulness in insomnia. As part of the screening process, potential subjects were evaluated by a physician dually board-certified in Sleep Medicine and Psychiatry (RMB) using a semi-structured sleep history and the Structured Clinical Interview for DSM-IV disorders (SCID).38 Insomnia subjects met research diagnostic criteria for insomnia disorder,3 as well as severity and duration criteria of sleep onset latency plus wake after sleep onset ≥ 30 min, frequency of ≥ 3 nights per week, and duration of symptoms ≥ 6 mo.39 Exclusion criteria were (1) current mood disorder or other significant neuropsychiatric condition based on the SCID; (2) evidence that the insomnia complaints were directly related to a medical condition or medication use; (3) inability or unwillingness to discontinue hypnotic or other psychotropic medication use for the duration of the study; and (4) presence of sleep apnea (apnea-hypopnea index > 10) or sleep related movement disorder (periodic limb movement arousal index > 10) based on in-laboratory polysomnography (see next paragraphs). Potential subjects also needed to be 18–65 years of age and right-handed. During the screening interview, insomnia participants were evaluated with the 17-item Hamilton Rating Scale for Depression (HRSD-17)40 to corroborate the exclusion of current major depression. In addition, insomnia participants completed various self-report questionnaires, including the: Insomnia Severity Index (ISI)41; Pittsburgh Sleep Quality Index (PSQI)42; Fatigue Severity Scale (FSS)43; and the Epworth Sleepiness Scale (ESS).44 Only subjects with an ISI > 11 and a PSQI > 5 were included in the analysis. General medical health was evaluated through the in-person screening interview and physical examination conducted by one of the coauthors (RMB).
Good Sleeper Controls
Age- and sex-matched control participants were drawn from a pool of right-handed control subjects selected for being good sleepers who either participated in a study on sleep homeostasis in depression (n = 6)45–49 or in a study on the effects of meditation (n = 2).50 Control subjects were recruited from the general population through publicly posted flyers, newspaper/ web advertisements, and email lists. They were screened for the presence of psychiatric disorders in the former case using a nonpatient SCID and medical history screen or in the latter case with a medical and psychiatric screening interview that included several questionnaires including the Quick Inventory of Depressive Symptoms51 and the Symptom Checklist-90-Revised52 to exclude current depression and other mental health issues. Exclusion criteria for control subject selection were (1) any current or past neuropsychiatric condition; (2) use of any psychotropic medication or medication that could affect sleep; and (3) evidence of insomnia or any other sleep disorder as defined previously.
General Protocol
Data were obtained from sleep studies performed at the Wisconsin Center for Sleep Medicine and Sleep Research. All subjects had an initial phone screening and a thorough in-person screening visit to determine eligibility, after which they were scheduled for their sleep study in the laboratory. Prior to their laboratory study, all subjects were asked to complete at least 1 w of sleep diaries and refrain from hypnotic agents or drugs that would affect sleep for at least 2 w. On the study night, participants arrived several hours before their usual bedtime and were outfitted with a high-density EEG cap. Subjects then participated in various waking recordings that were part of the larger study protocols. The studies involved various presleep waking recordings including standard eyes open/eyes closed conditions (all studies), auditory and visual evoked potentials (insomnia study), and/or attention and fear conditioning tasks (meditation). After these waking recordings, subjects were allowed to sleep undisturbed in the laboratory within 1 h of their usual bedtime. All subjects did waking tasks in the morning and some of the insomnia subjects (n = 3) participated in further waking recordings throughout the day. Control subjects were subsequently scheduled for other sleep nights as part of the parent protocol. The first laboratory night served as an adaptation/screening night and these data were used in the present analyses. All study procedures were approved by the Institutional Review Board of the University of Wisconsin-Madison.
Sleep Recordings
Participants underwent overnight in-laboratory high-density EEG recording sampled at 500 Hz and collected with vertex referencing (256 channels; Electrical Geodesics Inc., Eugene, OR). Lights out was within 1 h of the participants most consistently reported bedtime. Sleep staging was performed by a registered polysomnographic technologist according to standard American Academy of Sleep Medicine criteria using Alice Sleepware (Philips Respironics, Murrysville, PA) based on 30-sec epochs for 6 EEG channels at approximate 10–20 locations derived from the hdEEG array with bipolar re-referencing (F3/A2, F4/A1, C3/A2, C4/A1, O1/A2, O2/A1), electrooculography (EOG), and submental electromyography (EMG).53 Sleep disordered breathing was ruled out with concurrent polysomnography (n = 14) or nocturnal oximetry (two control subjects).
EEG Spectral Analysis
All EEG signals were collected at 500 Hz and high-pass filtered at 0.1 Hz. Prior to spectral analysis, each signal was downsampled to 128 Hz, band-pass filtered (two-way least-squares FIR, 1–40 Hz) in MATLAB (The MathWorks Inc., Natick, MA), re-referenced to the average of the scalp voltage for all 256 channels, and divided into consecutive 6-sec epochs. Semiautomatic artifact rejection procedures were utilized to remove channels and epochs with high-frequency noise or interrupted contact with the scalp, as in other recent studies.47,49,50,54 Specifically, thresholds were automatically calculated for low (1–4 Hz) and high (20–30 Hz) frequency ranges at the 99.8th and 99.5th percentile, respectively, for each channel. Spectral power in these ranges across all 6-sec NREM epochs for each channel were plotted and visually inspected. In cases where epochs with substantially greater low- or high-frequency power did not exceed the automatic threshold, the threshold was lowered and all epochs exceeding the threshold were removed. Channels with artifact affecting a majority of the recording were removed. Additional spectral-based and topo-graphic procedures were used to remove individual channels with distinctly greater power relative to neighboring channels. High-density electrode arrays make identifying such channels relatively obvious given that volume conduction effects make it unlikely that an individual electrode will vary a substantial amount from its neighbors unless it is unreliable. Importantly, insomnia and control groups did not differ in the portion of NREM epochs (77.8 ± 3.3% versus 80.52 ± 4.86%) or channels (93.7 ± 1.2% versus 94.5 ± 1.3%) retained following artifact rejection. Spectral analysis was performed using all clean 6-sec epochs within NREM sleep (Welch averaged modified periodogram with a Hamming window). To increase the signal-to-noise ratio, scalp analyses were restricted to inside channels falling within a plotting radius of 0.57 using the topoplot function of the EEGLAB plug-in for MATLAB55 and those that were good for at least five of the eight subjects in each group, yielding 170 channels overlaying the scalp. Remaining bad channels for each subject were interpolated using spline interpolation. For topographic analysis, we computed average spectral density for six frequency ranges (SWA/delta: 1–4Hz; theta: 4–8 Hz; alpha: 8–12 Hz; sigma: 12–15 Hz; beta: 15–25 Hz; and low gamma: 25–40 Hz) consistent with studies from our laboratory and others.56,57 Topographic maps of both absolute average referenced data and subject normalized (z-score across channels) were examined.
For comparison, similar spectral analysis (Welch method, Hamming window, 6-sec epochs) was also performed on the 5-minute broad-band filtered segment selected for source modeling (described in the next paragraphs). The same processing and thresholds were also applied to waking epochs that occurred during time in bed. Alpha peak frequency and amplitude were determined by using the MATLAB function detrend on the spectral data between 4–16 Hz before determining the peak amplitude between 6 and 14 Hz.
Source Modeling
Data for source modeling estimates were obtained by visually selecting for each subject a continuous 5-min segment that occurred during the relatively deepest part of NREM sleep for that subject. Segments were obtained primarily from staged N3 sleep during the first sleep cycle; however, if insufficient continuous N3 sleep was scored or there was no bout of deep sleep during the first sleep cycle, segments with some N2 and/ or during the second cycle were selected with a preference for taking the most continuous N3 epochs. For all participants, selected segments were within 3 h of sleep onset and were at least 1 min away from any noticeable arousal. Choosing relatively short duration continuous segments was critical for source imaging, making it possible to visually inspect the entire segment, and mitigating the possibility that arousal or muscle or eye movement activity contributed to any observable differences. Moreover, this data reduction approach ensured that an equal duration segment during a similar circadian time was compared for all subjects. Distributed source imaging is significantly improved by sampling as much of the sphere as possible,58,59 so each segment was reanalyzed for bad channels by visual inspection of both the raw and spectral density data to ensure that as many of the 256 available channels could be used in the source estimation. Identified bad channels were replaced by spline interpolation.
Source imaging was performed using GeoSource 2.0 (Electrical Geodesics Inc., Eugene, OR) on the previously described 5-min continuous segments of EEG data. As in previous studies,36,57,60 a four-shell head model derived from a magnetic resonance imaging scan from an individual whose head closely approximates the standard Montreal Neurological Institute (MNI) head and a standard set of coregistered electrode positions was used for the forward model. The inverse matrix was calculated via the minimum norm least-squares method with truncated singular value decomposition regularization at 10−1 for stabilization, and the standardized Low Resolution Brain Electromagnetic Tomography constraint (sLORETA61). The source space was limited to 2,447 cortical voxels (7 mm3 resolution), each assigned to a gyrus based on the MNI probabilistic atlas. Prior to source modeling, the original 0.1 Hz high-pass filtered data were broad-band-filtered in Net Station using a Kaiser window FIR filter between 1–40 Hz. Then, to examine the source of power for a particular frequency range of interest, the segments previously described were further band-pass filtered to the frequency range of interest in NetStation before source localization with GeoSource. A cortical estimate of power for each source voxel was computed by taking the average across the 5-min segment of the three-dimensional norm for each source voxel estimate. Alternatively, we also source modeled the broad-band filtered segments, computed the power spectral estimation in each of the three possible source directions for each source voxel, and then took the normative value across the dimension before averaging across the frequency range of interest, as has been done previously.57 Both methods yielded qualitatively similar results.
Statistical Analysis
Between-group differences in demographic and polysomnography variables, as well as global EEG power spectra, were evaluated with unpaired, two-tailed t-tests. A mixed model repeated-measures analysis of variance (ANOVA) was utilized for all six bands of interest (between-subjects factor group, within-subjects factor stage) to determine whether there was an interaction between group and stage that depended on frequency. All spectral density statistics were performed on log-normalized data. Scalp topography and source images were analyzed using statistical nonparametric mapping with supra-threshold cluster tests to correct for multiple comparisons.62 The cluster test uses a random shuffling of group assignment in all possible combinations (n = 12,870) and a t-value threshold (threshold = 2.3) to create a distribution of possible clusters sizes (i.e., groups of electrodes or source voxels above the chosen threshold). The cluster P value is then determined by comparison of the actual cluster size, the number of electrode or voxels with a cluster above threshold for the real subject groups, against the maximal cluster size distribution. Although this test faithfully addresses the problem of multiple comparisons across an image, it should also be noted that we did not attempt to strictly correct for the issue of multiple testing given the exploratory nature of this study. Correlations were assessed using Spearman rank correlation. Statistical analyses were performed using MATLAB and SPSS. All values are represented as mean ± standard error of the mean.
RESULTS
Demographic and Sleep Architecture Profile Was Consistent with Insomnia Diagnosis
Eight individuals with chronic insomnia and eight sex- and age-matched good sleeper control participants were included for analysis. Table 1 provides demographic and clinical characteristics for both insomnia and control groups. Insomnia participants demonstrated minimal levels of depression according to HRSD-17 scores, with positive ratings coming primarily from the three sleep items. Within the insomnia group, insomnia symptoms were of moderate severity (mean ISI 18.5 ± 1.1) with a chronic history (mean duration of complaints: 8.8 ± 2.4 years, range: 3–20 y). On the HRSD sleep measures, all eight participants reported a score of at least 1 on the 0–2 scale for early (onset) and middle (maintenance) sleep complaints and 7 of 8 for late-night sleep complaints. Mean scores across these items were similar (early: 1.50 ± 0.53; middle: 1.38 ± 0.52; late: 1.50 ± 0.76). Two subjects also endorsed symptoms of a general somatic nature (score of 1 on the 0–2 HRSD item scale and one of these also was rated as having mild somatic anxiety (score of 1). All of the insomnia subjects had previously taken some form of a benzodiazepine receptor agonist. Although three of the insomnia subjects stopped taking these medications 2 w prior to the study, most had not been taking them for over 1 y. One subject was taking melatonin until 2 w prior to the study.
Table 1.
Demographics and sleep architecture confirms insomnia profile.
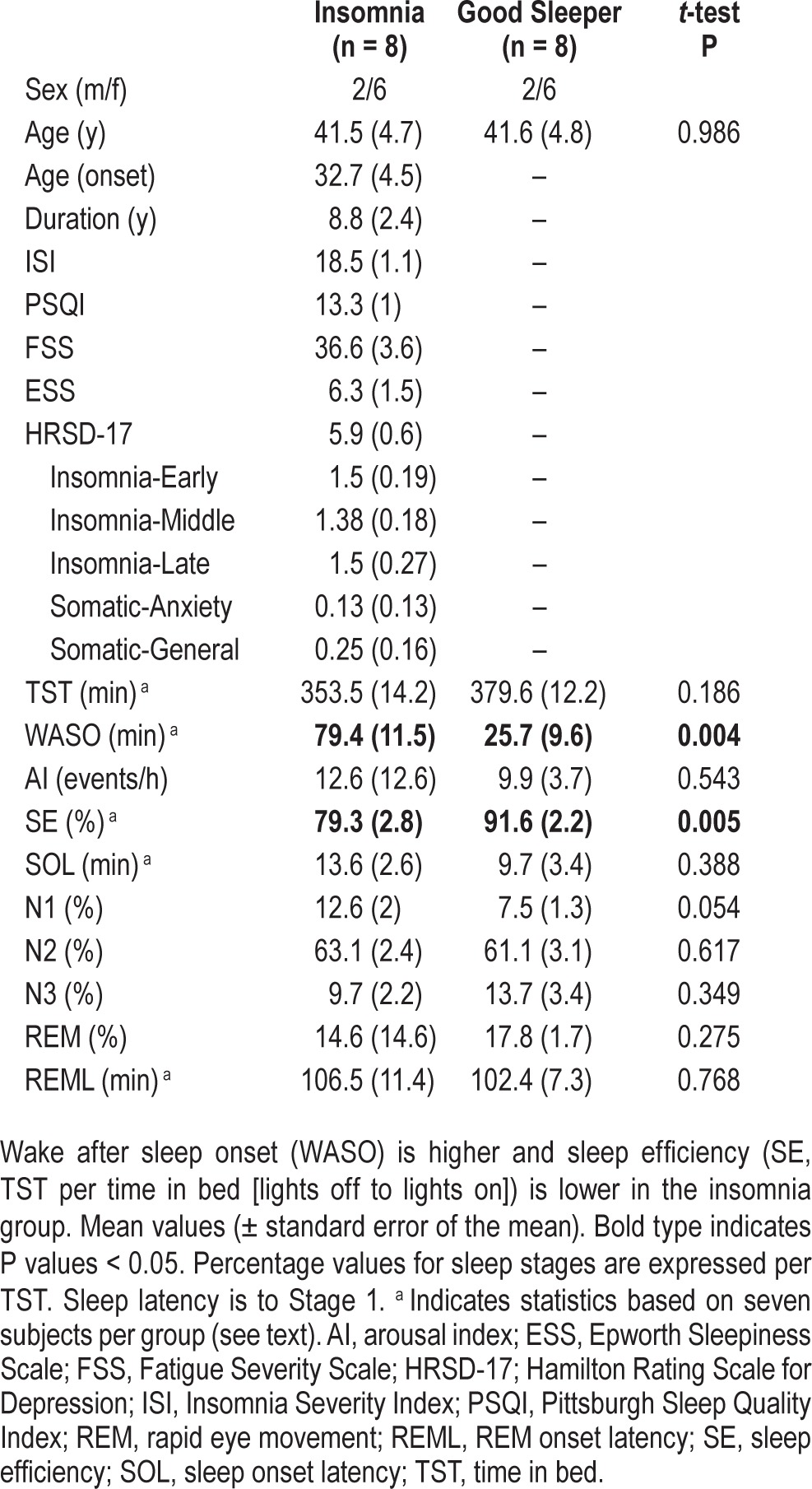
The sleep profile of insomnia participants in comparison to controls was consistent with previous studies. Compared to good sleeping controls, insomnia subjects exhibited significantly greater wake after sleep onset (WASO, P = 0.004) and lower sleep efficiency (P = 0.005). The proportion of total sleep time (TST) spent in stage N1 was higher for the insomnia group, although it failed to reach significance (P = 0.054). No other between-group significant differences were observed (Table 1). Due to technical difficulties during the start of two studies (one in each group), statistics for TST, wake after sleep onset (WASO), sleep efficiency (SE), sleep onset latency (SOL), and rapid eye movement latency (REML) were based on seven subjects in each group. Removal of these subjects did not substantially alter the age or sex matching because both were females of similar age (25 and 33 y old, respectively). Data from sleep diaries collected prior to the sleep night also suggested that insomnia subjects had more WASO, more awakenings, and greater SOL (Table S1, supplemental material).
Insomnia Subjects Had More Beta/Low Gamma During NREM Sleep than Good Sleeping Controls Globally
During NREM sleep, the insomnia group demonstrated significantly greater global EEG power (power spectral density averaged across all scalp channels) than good sleeping controls in a broad range of frequencies (Figure 1A). Interestingly, only slow wave (specifically < 5 Hz) and sigma (spindle) frequencies (specifically 11–16 Hz) were not different between groups for average power. When examined topographically, the difference between groups in the higher frequency bands (> 16 Hz) was spatially widespread (Figure 1B, beta and low gamma). There were 57 significantly different electrodes in one beta cluster (P = 0.02) and 19 channels in a cluster that trended toward significance (P = 0.06). The low gamma cluster was highly significant and widespread, accounting for 101 channels (P = 0.004). Notably, there were no significant changes in normalized topography for any frequency band (data not shown) which would suggest that the generators of different EEG rhythms do not differ between groups.
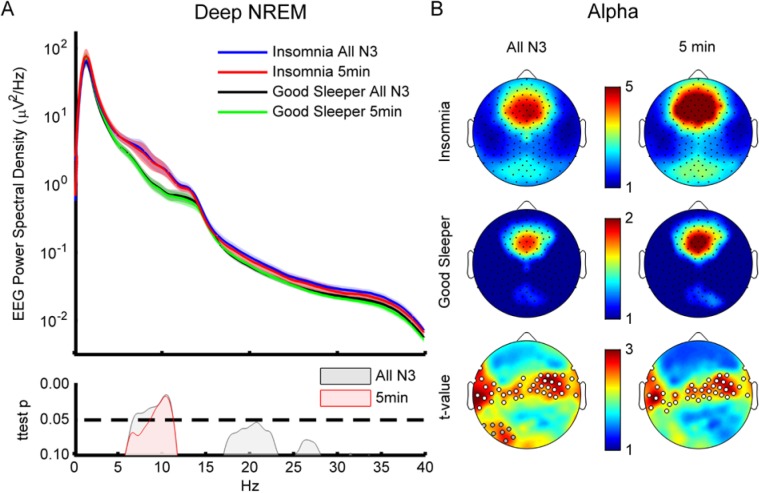
Figure 1.
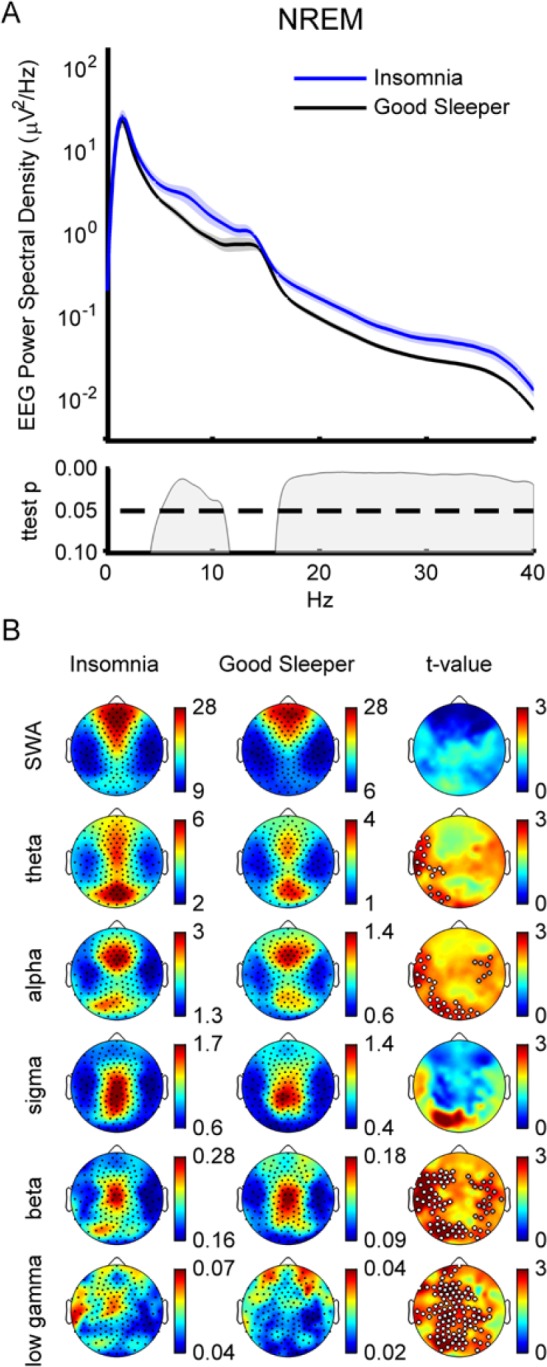
Whole night nonrapid eye movement (NREM). (A) Spectral density. Top graph is spectral power density average over all artifact free epochs during NREM sleep for insomnia subjects and controls. Shaded area represents standard error of the mean (SEM). Bottom graph shows P values. Insomnia subjects (blue line) show significantly more power than good sleeping controls (black line) between 5–11 Hz and above 16 Hz. Statistics based on logarithmically normalized data. (B) Topography. Spectral power density average across indicated frequency bands (SWA/delta: 1–4 Hz; theta: 4–8 Hz; alpha: 8–12 Hz; sigma: 12–15 Hz; beta: 15–25 Hz; and gamma: 25–40 Hz) for insomnia (left column) and control (middle column). Black dots are channels. Color bars are independently scaled to show similarity of topography between groups with maximum and minimum values shown to the right of each topoplot (units μV2/Hz). Right column shows individual channel t-maps. White dots indicate significance (P < 0.05) based on statistical nonparametric mapping cluster test. Gray dots show trend significance (P < 0.08).
Insomnia Subjects Had More Alpha in Sensory, Premotor, and Primary Motor Areas during Deep NREM Sleep Locally
The significantly higher power observed for lower frequencies (5–11 Hz, Figure 1A) in the insomnia compared to the control group during all-night NREM was not widespread across the scalp, but was localized to frontotemporal and occipital regions. The cluster in theta included 14 channels (P = 0.027) and the alpha band cluster included 28 channels (Figure 1B, P = 0.03). There were also smaller clusters with trend-level significance in both theta and alpha.
Given that N3 is (1) typically the most remote from wakefulness, with minimal contaminating muscle or eye movement activity, and (2) arguably the most homogenous sleep stage, being composed primarily of slow waves, we decided to specifically isolate N3 sleep epochs. When only N3 epochs were considered, the significant and spatially widespread group differences above 16 Hz were eliminated as can be seen in the lack of significant between group difference in the average spectral density at those frequencies (Figure 2A, black and blue lines). However, a significant between-group difference in average spectral density remained for the theta\alpha band (6.63–11.33 Hz, Figure 2A, gray significance curve). Topo-graphically, the significant between group difference in alpha became more apparent and involved two clusters of electrodes (n = 23, P = 0.034; n = 19, P = 0.038) extending from the left frontotemporal region bilaterally across the midline to the right frontotemporal region along with another cluster in the left occipital region that reached trend level significance (n = 8, P = 0.06, Figure 2B, column 1).
Figure 2.
Deep nonrapid eye movement (NREM) (N3 and continuous 5-min segments). (A) Spectral density. Same as Figure 1A except for only N3 epochs (blue and black lines). In addition, the data from the 5-min continuous segments are also shown for both the insomnia (red) and good sleeping controls (green). Bottom graph shows P values. The significant difference between subject groups for each comparison is similar except more confined to alpha for the segment data. (B) Alpha topography during sleep. Same as Figure 1B except only alpha band is shown (8–12 Hz). Left column is the average across subjects for only N3 epochs. Right column is the average for the continuous 5-min segment. Again, note similarity between the analysis methods (columns) and consistent differences between groups (cluster of white dots) regardless of method.
We then selected 5-min of the deepest available continuous NREM sleep in all subjects (see Methods) and examined the spectral profile and the topography of the selected segments. Of the 16 5-min segments selected, 12 were scored as entirely N3 sleep (six insomnia, six controls). Of the four remaining segments, the two segments from insomnia subjects both included over 3.5 min of staged N3 sleep, whereas the segments from the two control subjects included less than 1 min of N3. All but two of the segments were taken from the first NREM sleep episode.
Figure 2A demonstrates the close correspondence between the average spectral density of the segments and that of the all-night N3 data for both the insomnia (red and blue lines, respectively) and the control groups (green and black lines). Moreover, the segmented data also showed a significant difference in EEG spectral density between groups. This difference was similar to that observed in the all-night N3 data, but was slightly more confined to the alpha range (8.5–11.5 Hz, Figure 2A, pink significance curve). A similar agreement between segment and all night N3 data was observed when alpha scalp topography was compared between datasets (Figure 2B). Resembling the all-night data, there were two clusters of electrodes (n = 30, 45, P = 0.02, 0.013 respectively—note that only 41 of these are shown in Figure 2B scalp topography) demonstrating significant between-group difference that extended bilaterally from left to right through the central portion of the scalp. There was an 84% overlap of significant electrodes between the all-night and segment data.
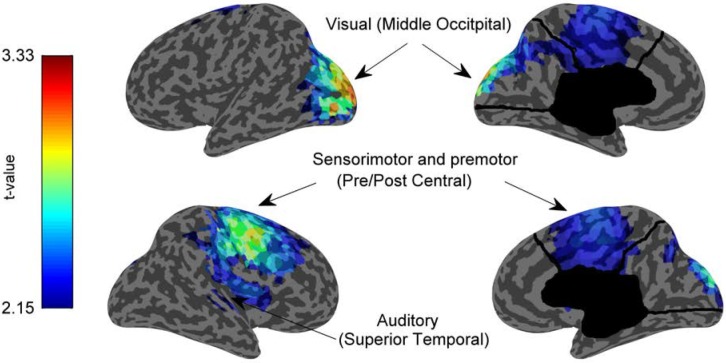
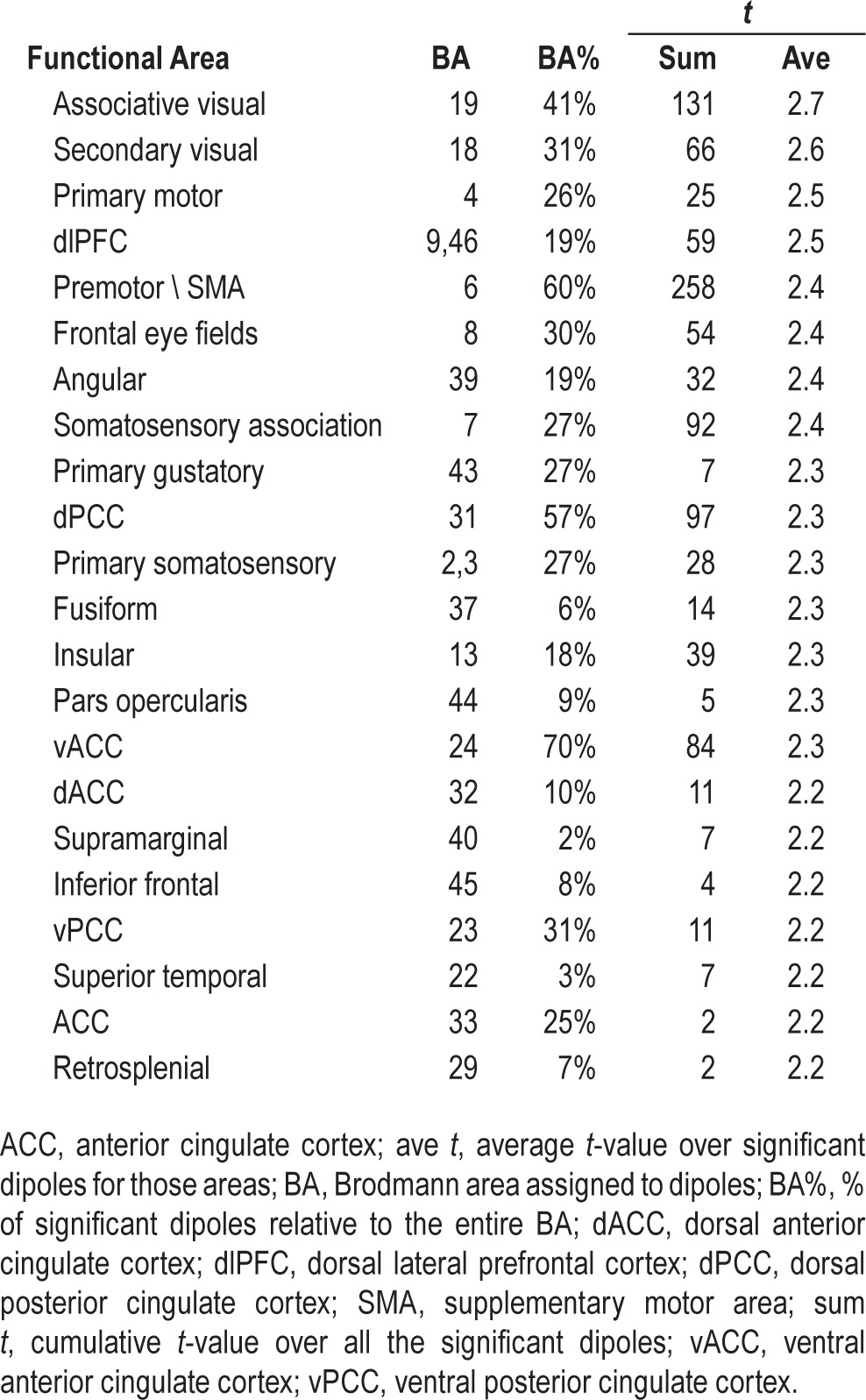
We next estimated the cortical sources of this circumscribed alpha difference between groups using the continuous data segments. Given our a priori interest in alpha from the scalp analysis, we filtered the segments in the alpha band (8–12 Hz, Kaiser) before source modeling the data. Source estimates for each segment were then averaged across subjects and compared between groups. Figure 3 shows the uncorrected t-values only for those voxels where the average source estimate of the alpha data for the insomnia group was significantly higher than that of the control group after the statistical nonparametric mapping cluster test (462 voxels, cluster P < 0.0406). Intriguingly, although varied, many of the areas with the most reliably higher alpha in insomnia subjects were sensory areas (including parts of the visual, auditory, and somatosensory cortices) as well as premotor and primary motor areas (Table 2). For comparison, we also examined the source estimation of high-frequency activity (> 16 Hz) in the segment data even though it was no longer significantly different between groups in the spectral analysis (data not shown). No significant differences were observed, which confirmed that the localized activity deviation was confined to the alpha range.
Figure 3.
Locally increased alpha in sensory and motor areas for insomnia compared to good sleeping control subjects during deep nonrapid eye movement (NREM) sleep. Inflated cortical map showing the T-values of the areas that were significantly different (P < 0.05) between insomnia and good sleeping control subjects using a statistical nonparametric mapping cluster test. Functional areas that are most significantly different include visual (Brodmann areas 18, 19), somatosensory (2, 3, 7, and 40) areas, motor (4) and premotor (6) areas. Auditory and language areas (22, 40) also show increases.
Table 2.
Significant dipoles ranked by average t-value.
As an exploratory analysis, we also examined artifact-free waking epochs that occurred while subjects were in bed. The majority of these epochs for both groups were during WASO (insomnia mean 86 ± 1.8%, control 72.8 ± 3.1%, not significant). Although there is substantially more wake epochs in the insomnia group (361 epochs ± 66 versus 69 ± 15) the results were similar when the same minimum number of epochs were used between groups. Alpha in the Wake during time in bed for both groups showed the predominant occipital alpha peak as would be expected during relaxed wakefulness (Figure S1A, supplemental material, right column). When compared to good sleeping controls, the insomnia subjects exhibited two temporoparietal areas of higher regional alpha activity (n = 17, P = 0.004; n = 12, P = 0.008). In addition, insomnia subjects also had more global theta activity than good sleeper controls during waking time in bed. No other frequency bands showed significant differences between insomnia and control groups. In order to determine how this waking alpha related to alpha during sleep, we estimated the peak alpha frequency (see Methods) in the four channels across the midline between Pz and Oz (channels 109, 118, 127, and 140) that exhibited the most alpha activity for both groups as well as calculated the amplitude of this peak as a measure of alpha power. Figure S1B suggests that the alpha frequency is more consistent in the insomnia group and the peaks are higher amplitude. When examining the correlations of the alpha peak amplitude with alpha power during sleep, there was a correlation between BA19 (Associate Visual Cortex, P = 0.032) one of the significant areas where alpha power during sleep was greater than controls but the correlation was not significant with BA6 (Premotor/Supplemental Motor Area, P = 0.1), another one of these areas. The relationship with these areas of alpha activity during sleep appeared to have been largely driven by the insomnia group as the waking alpha peak correlations were at trend level significance when only the insomnia subjects were considered (P = 0.07 and P = 0.056). The correlation dropped dramatically when the control group was examined independently. No correlation existed between alpha peak frequency and any of the areas of alpha during wake or sleep.
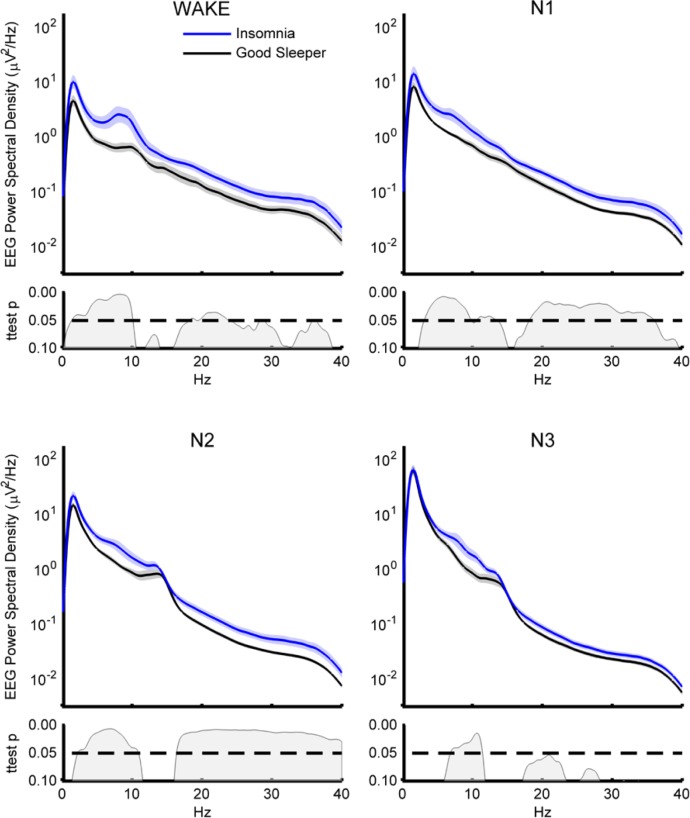
The persistence of lower frequency activity differences between the insomnia and control groups during waking and N3, along with the consistency of elevated power above 16 Hz when considering all NREM together (composed primarily of N2) suggested that there might be a group*stage interaction in terms of EEG power for different frequency ranges. However, when evaluating the effects of sleep stage on global power, independent mixed ANOVAs run for each frequency band (Group: insomnia, control; Stage: wake, N1, N2, N3) surprisingly demonstrated significant main effects of Group and Stage for all bands (group F > 4.72 P ≤ 0.047 stage F ≥ 4.43, P ≤ 0.039) but no interactions. The power spectral densities for all stage comparisons are plotted in Figure 4.
Figure 4.
EEG spectral density by stage. Power spectral density and P values categorized by sleep stage. Shaded area represents standard error of the mean (SEM). Statistics based on logarithmically normalized data. Wake stage is composed mostly of wake after sleep onset. EEG, electroencephalography.
DISCUSSION
In this study, we examined the sleep hdEEG of subjects in whom insomnia was diagnosed compared to good sleeping controls. We found that insomnia subjects had a global increase in high-frequency EEG activity (> 16 Hz) combined with a more circumscribed difference in theta (4–8 Hz) and alpha (8–12 Hz) power bands compared to good sleeping controls. When deep NREM sleep (N3) was examined, the high-frequency difference between groups diminished, whereas the higher regional alpha activity in insomnia subjects persisted. Furthermore, source imaging analysis demonstrated that sensory and sensorimotor cortical areas consistently exhibited elevated levels of alpha activity during deep NREM sleep in insomnia subjects relative to good sleeping controls.
The spectral density profile we observed during NREM— significantly higher power in the beta/low gamma range and less robust difference in the theta/alpha range—was consistent with data from previous limited channel studies (see the study by Buysse et al.20), especially given the larger proportion of females within our matched groups. Higher beta/low gamma in the insomnia group was more obvious in sleep stages N1 and N2 (Figure 4) similar to previously reported findings,19,25 although we found a less robust difference in sigma across all stages in contrast to findings elsewhere.19 The contrasting findings could be related to our small sample size, but it is also important to keep in mind that although these spectral findings are easiest to compare to other studies, the average power across all channels is a global measure and cannot always easily be compared to single-channel analyses.
More directly related to the focus of the current study, the whole night topographic analysis indicated that the change in beta/low gamma observed during NREM sleep in the global spectral analysis was relatively ubiquitous across the scalp, potentially affecting many different brain areas in the transition from wake toward sleep. The absolute and relative topography of slow waves and spindles, the waveforms most commonly associated with NREM sleep, were not different. Moreover, there were no significant differences in relative topography for any frequency band in any of our contrasting conditions, thus suggesting that the main sources of sleep related EEG activity were not different between insomnia and control subjects. Overall, these results are thus consistent with the idea of cortical arousal during sleep in insomnia. On the background of sleep specific waveforms rides high-frequency activity typically associated with wakefulness that may disrupt an insomnia patient's ability to achieve periods of deep and restorative slow wave sleep. Nonetheless, the utility of this interpretation and whether the heightened high frequency activity seen during NREM sleep relative to controls is unique to insomnia is still a matter of substantial debate that will require further investigation.
Alpha-Delta Sleep Revisited
As NREM sleep deepened, however, the influence of global high-frequency EEG power seemed to diminish in our sample. Indeed, the most intriguing and novel finding reported here is the regionally specific elevation in alpha EEG activity that occurred prominently during the deepest NREM sleep in insomnia subjects compared to controls. The disappearance of alpha activity is well known as the quintessential marker of the transition into sleep (stage N1). However, although first described over 40 y ago, the reappearance of alpha during slow wave sleep, so-called alpha-delta sleep, is still poorly understood. Alpha-delta sleep has been associated with chronic pain (i.e., fibromyalgia), chronic fatigue, and more generally with conditions involving nonrestorative sleep, including insomnia,23,63,64 and was recently reported to be a harbinger of future arousal.65 However, it has also been argued that the presence of alpha activity is not sufficient to produce nonrestorative sleep.66,67 Here, we describe higher alpha activity in a group of insomnia subjects that were neither included nor excluded from the study for the presence of alpha activity occurring during sleep. When this group was compared to age- and sex-matched good sleeping controls for average spectral density during N3 sleep, they nonetheless exhibited significantly more alpha activity. Although strong enough to show up in the global average, it was apparent that the between-group difference observed in alpha power had a specific scalp topography, suggesting that not all cortical areas were equally affected. The use of hdEEG allowed us to examine the cortical source of this between-group discrepancy. Source imaging revealed that the areas where alpha activity in insomnia was most reliably elevated during deep NREM sleep when compared to good sleeping controls were cortical areas that included several sensory domains as well as sensorimotor/motor areas.
Evidence for Local Sleep Dysregulation?
It is tempting to suggest that in insomnia subjects, sensory areas that should have been asleep, like the rest of the brain, instead showed classic signs of wakefulness.35 Extending this interpretation, one might argue that higher frequency activity, thought to represent cognitive processing,50,68,69 actually persists into early NREM sleep, whereas only the lower frequency oscillations similar to alpha, representing rudimentary sensory perception and/or startle, occur during deep slow wave sleep. Although intriguing, such conclusions are premature at this point. It is not entirely clear that the alpha activity observed during sleep is the same as that which is characteristic of relaxed wakefulness. Although our exploratory analysis provided some evidence for a relationship between alpha in different states, the topography of alpha during NREM sleep, with a frontocentral maximum (Figure 2B), is decidedly unlike the dominant occipital alpha hotspot seen in wakefulness (Figure S1). Therefore, it is likely that the main generators as well as the functions of the alpha rhythm are different between states.67 Regardless of the exact functional implications of the activity these sensory and motor areas were exhibiting during sleep in insomnia patients, it was clearly different from that observed in good sleeping controls. Whether this means these areas were maintaining a connection with the environment, lowering the threshold for outside stimuli to lighten sleep, or whether it means they were continuing in an activity mode that makes sleep ultimately non-restorative, will undoubtedly need to be tested. Intriguingly, evidence from both functional magnetic resonance imaging and EEG event-related potential studies have suggested that enhanced sensory-motor processing may persist into sleep due to maintained activation and/or diminished inhibitory control in insomnia subjects.70–72 Our study suggests that this altered sensory motor activity may persist even into the deepest stages of NREM sleep and provides compelling evidence for a recently hypothesized model of localized sleep dysregulation in insomnia.35
Study Limitations
Because this was a pilot study with a limited number of subjects, these results may not necessarily be generalizable to all patients with chronic insomnia. In addition, the fact that sleep EEG data from control subjects was acquired under separate protocols meant that differences in the presleep tasks or some other unknown differences between studies could have contributed to our findings. Moreover, the studies did not use identical questionnaires or assessments, so insomnia and control subjects could not be directly compared on all relevant measures.
Another potential limitation of this study is the role of pain. Indeed, given the association of alpha-delta sleep and pain73,74 and our unanticipated finding of increased local alpha during deep sleep, the lack of extensive group profiling on acute and chronic pain is unfortunate. However, it is noteworthy that none of the patients received a pain disorder diagnosis or was being treated for chronic pain. Other potential caveats include the lack of actigraphy monitoring in participants to confirm sleep history prior to the EEG recordings as well as the single-night recording design of this pilot study, which did not allow controlling for potential first-night effects. Given that prior literature suggests that such effects may or may not be minimized with spectral analysis approaches,75,76 future studies would benefit from at least 2 nights of recordings. Furthermore, while groups were age- and sex-matched in this study, potential differences in the effects between men and women also limit the generalizability of these results.20 Because the small sample size here does not allow for appropriate evaluation of sex effects, future studies would benefit from incorporating this consideration into the study design. Finally, we did not have a well enough controlled experimental design to examine the time course of waking EEG to determine how presleep alpha activity compares to alpha seen during sleep.
Conclusion and Future Directions
Despite these limitations, we believe these results highlight the importance of examining local sleep-wake dysregulation in specific brain regions using high-density EEG. Only one other study appears to have evaluated the topographical distribution of high-frequency activity in chronic insomnia during NREM sleep and their main focus was on the transition to sleep.77 Future studies assessing a larger number of insomnia patients with varied clinical symptoms (e.g., sleep onset versus maintenance insomnia, paradoxical insomnia, etc.), as well as other medical and psychiatric comorbidities, will be required to determine whether these local changes in brain activity during sleep are specific to certain patient populations or reflect a shared neurobiologic finding in insomnia. Moreover, performing experimental manipulations in healthy individuals (i.e., serial nocturnal awakenings)60,65,78 while monitoring the activity of these areas during the transitions between wakefulness and sleep could help reveal their contribution to the spontaneous awakenings occurring in individuals with sleep disturbances as well as determine the extent to which these brain areas are involved in local wakefulness. A similar approach could also be utilized in studies employing pharmacological compounds aimed to reduce sleep fragmentation in insomniac patients. Altogether, these approaches, combined with other neuroimaging techniques, will hopefully continue to shed a brighter light on the neurobiological basis of insomnia.
DISCLOSURE STATEMENT
This was not an industry supported study. This research was funded by the UW Foundation and Wisconsin Partnership Program's Medical Education and Research Committee (MERC), the NIH Mental Health Grant P20MH077967, and the NIH National Center for Complementary and Alternative Medicine Grant P01AT004952. Dr. Benca has consulted for Merck, Jazz and Janssen and has received research support from Merck. Dr. Rumble has received research support from Merck. Dr. Tononi has consulted for Philips Healthcare who endowed the David P. White Chair in Sleep Medicine, held by Dr. Tononi at the University of Wisconsin-Madison. Dr. Tononi has received research support from Philips Healthcare and has been a symposium speaker for Philips Healthcare and Sanofi. Dr. Riedner receives partial salary support from the Merck and Philips Healthcare Grants held by Drs. Benca and Tononi and, along with Dr. Tononi, is listed on several joint patent applications between the Wisconsin Alumni Research Foundation and Philips Healthcare related to sleep technology. Dr. Plante has received royalties from Cambridge University Press. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We gratefully acknowledge the contributions of the following individuals who assisted in subject recruitment and data collection of control subjects: Eric Landsness, Michael J. Peterson, David Bachhuber, Daniela Dentico, Corinna Zennig, and Antoine Lutz. We also thank Stephanie Jones for helpful discussions on data analysis and the manuscript.
REFERENCES
- 1.Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999;22:S347–53. [PubMed] [Google Scholar]
- 2.Morin CM, LeBlanc M, Daley M, Gregoire JP, Mérette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123–30. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Edinger J, Bonnet M, Bootzin R. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Mendoza J, Vgontzas AN. Insomnia and its impact on physical and mental health. Curr Psychiatry Rep. 2013;15:418. doi: 10.1007/s11920-013-0418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palagini L, Bruno RM, Gemignani A, Baglioni C, Ghiadoni L, Riemann D. Sleep loss and hypertension: a systematic review. Curr Pharm Des. 2013;19:2409–19. doi: 10.2174/1381612811319130009. [DOI] [PubMed] [Google Scholar]
- 6.Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30:213–8. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- 7.Kessler RC, Berglund PA, Coulouvrat C, et al. Insomnia and the performance of US workers: results from the America insomnia survey. Sleep. 2011;34:1161–71. doi: 10.5665/SLEEP.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnet MH, Burton GG, Arand DL. Physiological and medical findings in insomnia: implications for diagnosis and care. Sleep Med Rev. 2014;18:111–22. doi: 10.1016/j.smrv.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Maes J, Verbraecken J, Willemen M, et al. Sleep misperception, EEG characteristics and autonomic nervous system activity in primary insomnia: a retrospective study on polysomnographic data. Int J Psychophysiol. 2014;91:163–71. doi: 10.1016/j.ijpsycho.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Farina B, Dittoni S, Colicchio S, et al. Heart rate and heart rate variability modification in chronic insomnia patients. Behav Sleep Med. 2014;12:290–306. doi: 10.1080/15402002.2013.801346. [DOI] [PubMed] [Google Scholar]
- 13.Vgontzas AN, Bixler EO, Lin HM, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86:3787–94. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- 14.Bonnet MH, Arand DL. Insomnia, metabolic rate and sleep restoration. J Intern Med. 2003;254:23–31. doi: 10.1046/j.1365-2796.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 15.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–8. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 16.Nofzinger EA, Buysse DJ, Miewald JM, et al. Human regional cerebral glucose metabolism during non-rapid eye movement sleep in relation to waking. Brain. 2002;125:1105–15. doi: 10.1093/brain/awf103. [DOI] [PubMed] [Google Scholar]
- 17.Cervena K, Espa F, Perogamvros L, Perrig S, Merica H, Ibanez V. Spectral analysis of the sleep onset period in primary insomnia. Clin Neurophysiol. 2014;125:979–87. doi: 10.1016/j.clinph.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 18.St-Jean G, Turcotte I, Pérusse AD, Bastien CH. REM and NREM power spectral analysis on two consecutive nights in psychophysiological and paradoxical insomnia sufferers. Int J Psychophysiol. 2013;89:181–94. doi: 10.1016/j.ijpsycho.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Spiegelhalder K, Regen W, Feige B, et al. Increased EEG sigma and beta power during NREM sleep in primary insomnia. Biol Psychol. 2012;91:329–33. doi: 10.1016/j.biopsycho.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Buysse DJ, Germain A, Hall ML, et al. EEG spectral analysis in primary insomnia: NREM period effects and sex differences. Sleep. 2008;31:1673–82. doi: 10.1093/sleep/31.12.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marzano C, Ferrara M, Sforza E, De Gennaro L. Quantitative electroencephalogram (EEG) in insomnia: a new window on pathophysiological mechanisms. Curr Pharm Des. 2008;14:3446–55. doi: 10.2174/138161208786549326. [DOI] [PubMed] [Google Scholar]
- 22.Staner L, Cornette F, Maurice D, et al. Sleep microstructure around sleep onset differentiates major depressive insomnia from primary insomnia. J Sleep Res. 2003;12:319–30. doi: 10.1046/j.0962-1105.2003.00370.x. [DOI] [PubMed] [Google Scholar]
- 23.Krystal AD, Edinger JD, Wohlgemuth WK, Marsh GR. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002;25:630–40. [PubMed] [Google Scholar]
- 24.Perlis ML, Smith MT, Andrews PJ, Orff H, Giles DE. Beta/Gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24:110–7. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]
- 25.Perlis ML, Kehr EL, Smith MT, Andrews PJ, Orff H, Giles DE. Temporal and stagewise distribution of high frequency EEG activity in patients with primary and secondary insomnia and in good sleeper controls. J Sleep Res. 2001;10:93–104. doi: 10.1046/j.1365-2869.2001.00247.x. [DOI] [PubMed] [Google Scholar]
- 26.Merica H, Blois R, Gaillard JM. Spectral characteristics of sleep EEG in chronic insomnia. Eur J Neurosci. 1998;10:1826–34. doi: 10.1046/j.1460-9568.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- 27.Merica H, Gaillard JM. The EEG of the sleep onset period in insomnia: a discriminant analysis. Physiol Behav. 1992;52:199–204. doi: 10.1016/0031-9384(92)90258-4. [DOI] [PubMed] [Google Scholar]
- 28.Freedman RR. EEG power spectra in sleep-onset insomnia. Electroencephalogr Clin Neurophysiol. 1986;63:408–13. doi: 10.1016/0013-4694(86)90122-7. [DOI] [PubMed] [Google Scholar]
- 29.Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6:179–88. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 30.Hung C-S, Sarasso S, Ferrarelli F, et al. Local experience-dependent changes in the wake EEG after prolonged wakefulness. Sleep. 2013;36:59–72. doi: 10.5665/sleep.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy M, Huber R, Esser S, et al. The cortical topography of local sleep. Curr Top Med Chem. 2011;11:2438–46. doi: 10.2174/156802611797470303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 33.Krueger JM, Rector DM, Roy S, Van Dongen HP a, Belenky G, Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci. 2008;9:910–9. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nobili L, De Gennaro L, Proserpio P, et al. Local aspects of sleep: observations from intracerebral recordings in humans. Prog Brain Res. 2012;199:219–32. doi: 10.1016/B978-0-444-59427-3.00013-7. [DOI] [PubMed] [Google Scholar]
- 35.Buysse DJ, Germain A, Hall M, Monk TH, Nofzinger EA. A neurobiological model of insomnia. Drug Discov Today Dis Model. 2011;8:129–37. doi: 10.1016/j.ddmod.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy M, Riedner BA, Huber R, Massimini M, Ferrarelli F, Tononi G. Source modeling sleep slow waves. Proc Natl Acad Sci U S A. 2009;106:1608–13. doi: 10.1073/pnas.0807933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spiegelhalder K, Regen W, Baglioni C, Riemann D, Winkelman JW. Neuroimaging studies in insomnia. Curr Psychiatry Rep. 2013;15:405. doi: 10.1007/s11920-013-0405-0. [DOI] [PubMed] [Google Scholar]
- 38.First MB, Spitzer RL, Gibbon M, Williams JBW. New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) [Google Scholar]
- 39.Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Quantitative criteria for insomnia. Behav Res Ther. 2003;41:427–45. doi: 10.1016/s0005-7967(02)00023-2. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morin CM. New York, NY: Guilford Press; 1993. Insomnia: Psychological Assessment and Management. [Google Scholar]
- 42.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 43.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–3. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 44.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 45.Landsness EC, Goldstein MR, Peterson MJ, Tononi G, Benca RM. Antidepressant effects of selective slow wave sleep deprivation in major depression: a high-density EEG investigation. J Psychiatr Res. 2011;45:1019–26. doi: 10.1016/j.jpsychires.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plante DT, Goldstein MR, Landsness EC, et al. Topographic and sex-related differences in sleep spindles in major depressive disorder: a high-density EEG investigation. J Affect Disord. 2013;146:120–5. doi: 10.1016/j.jad.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldstein MR, Plante DT, Hulse BK, et al. Overnight changes in waking auditory evoked potential amplitude reflect altered sleep homeostasis in major depression. Acta Psychiatr Scand. 2012;125:468–77. doi: 10.1111/j.1600-0447.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Plante DT, Landsness EC, Peterson MJ, et al. Altered slow wave activity in major depressive disorder with hypersomnia: a high density EEG pilot study. Psychiatry Res. 2012;201:240–4. doi: 10.1016/j.pscychresns.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plante DT, Landsness EC, Peterson MJ, et al. Sex-related differences in sleep slow wave activity in major depressive disorder: a high-density EEG investigation. BMC Psychiatry. 2012;12:146. doi: 10.1186/1471-244X-12-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferrarelli F, Smith R, Dentico D, et al. Experienced mindfulness meditators exhibit higher parietal-occipital EEG gamma activity during NREM sleep. PLoS One. 2013;8:e73417. doi: 10.1371/journal.pone.0073417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 52.Derogatis L, Unger R. Symptom Checklist-90-Revised. Corsini Encyclopedia of Psychology. 2010 [Google Scholar]
- 53.Iber C, Ancoli-Israel S, Chesson A, Quan S for the American Academy of Sleep Medicine. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. [Google Scholar]
- 54.Jones SG, Riedner B a, Smith RF, et al. Regional reductions in sleep electroencephalography power in obstructive sleep apnea: a high-density EEG study. Sleep. 2014;37:399–407. doi: 10.5665/sleep.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 56.Kurth S, Ringli M, Geiger A, LeBourgeois M, Jenni OG, Huber R. Mapping of cortical activity in the first two decades of life: a high-density sleep electroencephalogram study. J Neurosci. 2010;30:13211–9. doi: 10.1523/JNEUROSCI.2532-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murphy M, Bruno M, Riedner BA, et al. Propofol anesthesia and sleep: a high-density EEG study. Sleep. 2011;34:283–91. doi: 10.1093/sleep/34.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, Grave de Peralta R. EEG source imaging. Clin Neurophysiol. 2004;115:2195–222. doi: 10.1016/j.clinph.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 59.Song J, Davey C, Poulsen C, et al. EEG source localization: sensor density and head surface coverage. J Neurosci Methods. 2015;256:9–21. doi: 10.1016/j.jneumeth.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 60.Riedner BA, Hulse BK, Murphy MJ, Ferrarelli F, Tononi G. Temporal dynamics of cortical sources underlying spontaneous and peripherally evoked slow waves. Prog Brain Res. 2011;193:201–18. doi: 10.1016/B978-0-444-53839-0.00013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol. 2002;24(Suppl D):5–12. [PubMed] [Google Scholar]
- 62.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hauri P, Hawkins DR. Alpha-delta sleep. Electroencephalogr Clin Neurophysiol. 1973;34:233–7. doi: 10.1016/0013-4694(73)90250-2. [DOI] [PubMed] [Google Scholar]
- 64.Martinez D, Breitenbach TC, Lenz MDCS. Light sleep and sleep time misperception - relationship to alpha-delta sleep. Clin Neurophysiol. 2010;121:704–11. doi: 10.1016/j.clinph.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 65.McKinney SM, Dang-Vu TT, Buxton OM, Solet JM, Ellenbogen JM. Covert waking brain activity reveals instantaneous sleep depth. PLoS One. 2011;6:e17351. doi: 10.1371/journal.pone.0017351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stone KC, Taylor DJ, McCrae CS, Kalsekar A, Lichstein KL. Nonrestorative sleep. Sleep Med Rev. 2008;12:275–88. doi: 10.1016/j.smrv.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 67.Pivik R, Harman K. A reconceptualization of EEG alpha activity as an index of arousal during sleep: all alpha activity is not equal. J Sleep Res. 1995;4:131–7. doi: 10.1111/j.1365-2869.1995.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 68.Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–16. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- 69.Pulvermüller F, Birbaumer N, Lutzenberger W, Mohr B. High-frequency brain activity: its possible role in attention, perception and language processing. Prog Neurobiol. 1997;52:427–45. doi: 10.1016/s0301-0082(97)00023-3. [DOI] [PubMed] [Google Scholar]
- 70.Killgore WDS, Schwab ZJ, Kipman M, Deldonno SR, Weber M. Insomnia-related complaints correlate with functional connectivity between sensory-motor regions. Neuroreport. 2013;24:233–40. doi: 10.1097/WNR.0b013e32835edbdd. [DOI] [PubMed] [Google Scholar]
- 71.Bastien CH, St-Jean G, Morin CM, Turcotte I, Carrier J. Chronic psychophysiological insomnia: hyperarousal and/or inhibition deficits? An ERPs investigation. Sleep. 2008;31:887–98. doi: 10.1093/sleep/31.6.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cortoos A, De Valck E, Pattyn N, Mairesse O, Cluydts R. Excitatory versus inhibitory impairments in insomnia patients: an ERP study. Int J Psychophysiol. 2014;93:62–9. doi: 10.1016/j.ijpsycho.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 73.Horne JA, Shackell BS. Alpha-like EEG activity in non-REM sleep and the fibromyalgia (fibrositis) syndrome. Electroencephalogr Clin Neurophysiol. 1991;79:271–6. doi: 10.1016/0013-4694(91)90122-k. [DOI] [PubMed] [Google Scholar]
- 74.Moldofsky H, Scarisbrick P, England R, Smythe H. Musculosketal symptoms and non-REM sleep disturbance in patients with “fibrositis syndrome” and healthy subjects. Psychosom Med. 37:341–51. doi: 10.1097/00006842-197507000-00008. [DOI] [PubMed] [Google Scholar]
- 75.Curcio G, Ferrara M, Piergianni A, Fratello F, De Gennaro L. Paradoxes of the first-night effect: a quantitative analysis of antero-posterior EEG topography. Clin Neurophysiol. 2004;115:1178–88. doi: 10.1016/j.clinph.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 76.Israel B, Buysse DJ, Krafty RT, Begley A, Miewald J, Hall M. Short-term stability of sleep and heart rate variability in good sleepers and patients with insomnia: for some measures, one night is enough. Sleep. 2012;35:1285–91. doi: 10.5665/sleep.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Corsi-Cabrera M, Figueredo-Rodríguez P, del Río-Portilla Y, Sánchez-Romero J, Galán L, Bosch-Bayard J. Enhanced frontoparietal synchronized activation during the wake-sleep transition in patients with primary insomnia. Sleep. 2012;35:501–11. doi: 10.5665/sleep.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Siclari F, Bernardi G, Riedner BA, LaRocque JJ, Benca RM, Tononi G. Two distinct synchronization processes in the transition to sleep: a high-density electroencephalographic study. Sleep. 2014;37:1621–37. doi: 10.5665/sleep.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.