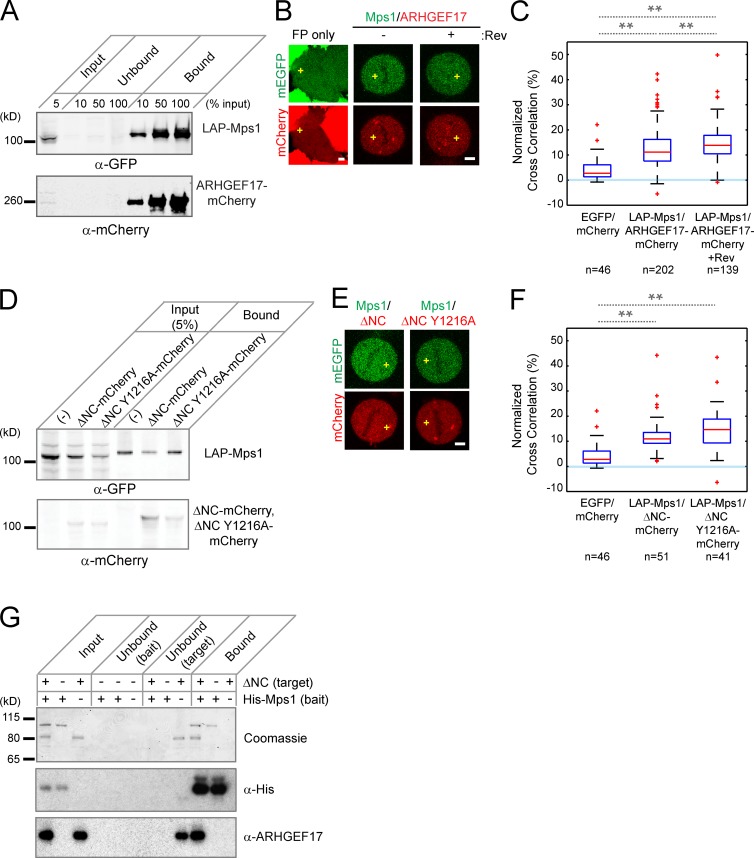
Figure 4.
ARHGEF17 and Mps1 interact during mitosis. (A) Coimmunoprecipitation of ARHGEF17 with Mps1: LAP-tagged Mps1 (LAP-Mps1) and mCherry-tagged ARHGEF17 (ARHGEF17-mCherry) were immunoprecipitated using GFP-binding protein coupled to agarose beads. Input, supernatants (Unbound), and immunoprecipitates (Bound) were analyzed by Western Blot (anti-GFP and anti-mCherry). (B and C) FCCS of Mps1 and ARHGEF17. Exemplary cells (B; yellow crosses mark position for FCCS measurement) and normalized cross-correlation (C) of ARHGEF17-mCherry and LAP-Mps1 with or without reversine treatment of >40 cells (specific numbers indicated)/three independent experiments. (D) Coimmunoprecipitation of ARHGEF17 fragments with Mps1: LAP-Mps1 and mCherry-tagged ARHGEF17 fragments (ΔNC-mCherry and ΔNC Y1216A-mCherry) were precipitated using GFP-binding protein coupled to agarose beads. Input and precipitates were analyzed by Western blot (anti-GFP and anti-mCherry). (E and F) FCCS of Mps1 and ARHGEF17 fragments. Exemplary cells (E) and normalized cross-correlation (F) of LAP-Mps1 and ARHGEF17 fragments (ΔNC-mCherry and ΔNC Y1216A-mCherry) of >40 cells (specific numbers indicated)/three independent experiments. (G) In vitro pull-down of ARHGEF17 fragments with Mps1. His-tagged Mps1 (bait) and untagged ARHGEF17 fragments (ΔNC; target) were precipitated using His-tag binding protein coupled to Talon beads. Input, supernatants (Unbound), and precipitates (Bound) were analyzed by Western blot (anti–His-tag [middle] and anti-ARHGEF17 [bottom]). Coomassie brilliant blue staining was used as internal protein control in each condition. Boxes show median, 25–75%; whiskers show 1.5× interquartile range. **, P < 0.01 by two-tailed unpaired Student’s t test, compared with mEGFP and mCherry (C and F) or between metaphase and metaphase with reversine treatment (F). Slower migration of Mps1/ARHGEF17 bands could be caused by phosphorylation (A and D). Bars, 5 µm.