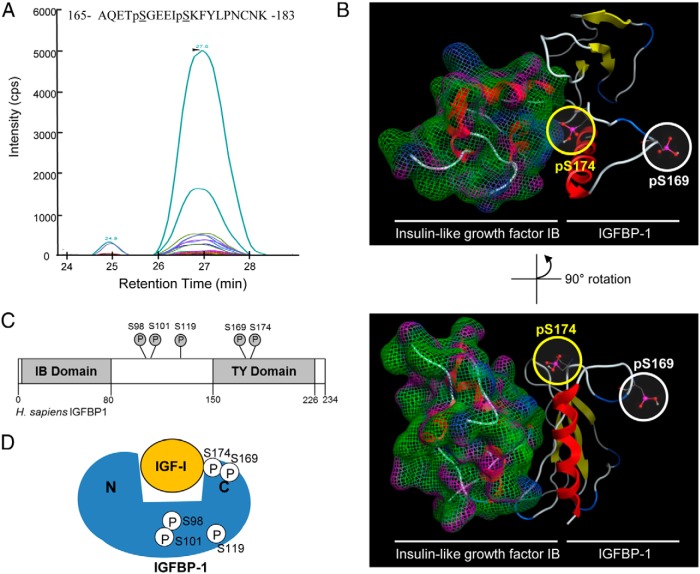
Figure 7.
Discovery of IGFBP-1 (Ser174) phosphorylation. MRM transitions used for the discovery and detection of dual phosphorylation at Ser169 and Ser174. Colored traces represent the detection and specificity of each transition ion generated specifically from the dual phosphorylated IGFBP-1 at Ser169 and Ser174 (see Supplemental Table 2 for transition list). B, Molecular modeling of the IGFBP C terminus and IGF-IB complex, based on the available crystal structure (2DSQ). Both the phosphorylated Ser174 and Ser169 residues are highlighted. The C terminus of IGFBP-1 was modeled from the available PDB 2DSQ:G crystal structure. C, Schematic of IGFBP-1 and phosphorylation sites (Ser98, Ser101, Ser119, Ser169, and Ser174) in proximity to the IGFBP-1-IGF-binding (IB) and TY domains. Phosphorylation sites Ser98, Ser101, and Ser119 are found within the interdomain region, whereas both Ser169 and Ser174 are within the TY domain. D, Proximity of the studied phosphorylation sites within the IGFBP-1 protein, relative to each other and to the IGF-I-binding site.