Figure 4.
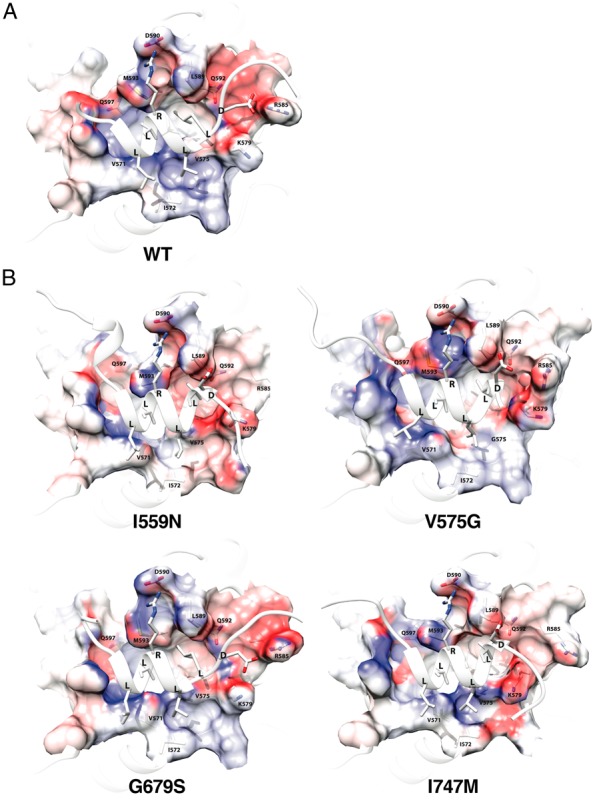
Four representative pathologic GRα mutants change conformational and chemical properties of their AF-2 surface. The AF-2 surface of wild-type GRα (A) and 4 representative pathologic GRα mutants (I559N, ligand-binding defective; V575G, AF-2 defective; G679S and I747M, both defective) (B) are shown. Positive and negative electrostatic potential are indicated with blue and red, respectively. Key residues of the receptors and the LXXLL peptide that make important molecular interactions are incorporated. The structures shown and their calculated biochemical properties are those of the averaged trajectories. WT, wild type.
