Abstract
Fractionation of geopropolis from Melipona scutellaris, guided by antiproliferative activity against two colon cancer cell lines (COLO205 and KM12), led to the isolation of two new cinnamic acid esters, mammea-type coumarins 5,7-dihydroxy-6-(3-methyl-2-butenyl)-8-(4-cinnamoyl-3-methyl-1-oxobutyl)-4-propyl-coumarin (1) and 5,7-dihydroxy-6-(4-cinnamoyl-3-methyl-1-oxobutyl)-4-phenylcoumarin (2), along with five known coumarins, mammeigin (3), hydroxymammeigin (4), mammeisin (5), cinnamoyloxy-mammeisin (6), and mammein (7), and the prenylated benzophenone ent-nemorosone (8). Among the isolated compounds, 5 and 7 showed the highest cell growth inhibition against COLO205 (GI50 9.7 and 10.7 μM, respectively) and KM12 (GI50 12.0 and 10.9 μM, respectively). The presence of these compounds suggests that plants of Clusiaceae family, especially the genera Kielmeyera and Clusia, are likely to be major sources of geopropolis produced by M. scutellaris.
Keywords: Clusiaceae, geopropolis, Melipona scutellaris, cytotoxity, coumarins
Introduction
Propolis, a resin collected by bees from several plants, has been reported to possess a great variety of biological activities. The wide range of activities is a consequence of its complex chemical composition, which can vary according to plant source, season, and bee species [1, 2]. Most of the studies available in the international literature concern propolis collected by Apis mellifera, whereas other types of propolis collected by different species of bees have been sparsely studied. Melipona scutellaris Latreille 1811, a native Brazilian stingless bee, is an important pollinator and recently has been considered threatened. This primitive bee produces a different type of propolis made of plant resins, wax, and soil, called geopropolis [3]. Our group has previously demonstrated a promising range of biological activities including anti-inflammatory [4], antimicrobial as well as antiproliferative [5]. Our previous studies have suggested the presence of cinnamic acid derivatives as well as prenylated compounds in this geopropolis [5], although no study has yet described the chemical composition of geopropolis from M. scutellaris. Therefore, a bioassay-guided fractionation and isolation based on the antiproliferative activity against colon cancer cell lines was undertaken, which yielded two new compounds and six known compounds. The compounds were tested in the NCI 60-cell screen in order to assess their cytotoxic profile.
Results and Discussion
In order to isolate and identify the compounds present in geopropolis, we carried out a fractionation of the ethanolic extract of geopropolis (EEGP) from M. scutellaris guided by growth inhibitory activity against the colon cancer cell lines COLO205 and KM12. Bioguided fractionation of the EEGP using diol, Sephadex LH-20, and normalphase HPLC separation led to the isolation of one new 4-propyl coumarin (1), one new 4-phenyl coumarin (2), five known coumarins (3–7), and one known benzophenone (8) (Fig. 1). The structures were determined by spectroscopic analysis, including 1D and 2D NMR (COSY, HSQC and HMBC) and HRESIMS experiments. The structures of the known compounds were determined by comparing their spectroscopic data with the literature.
Fig. 1.
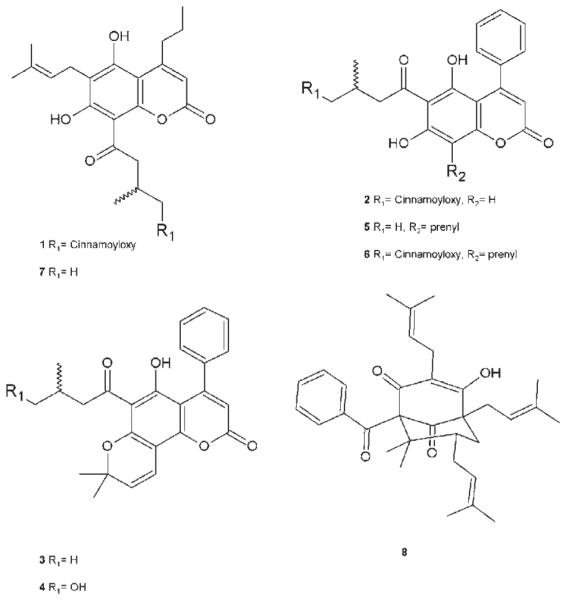
Chemical structures of compounds 1–8 isolated from M. scutellaris geopropolis.
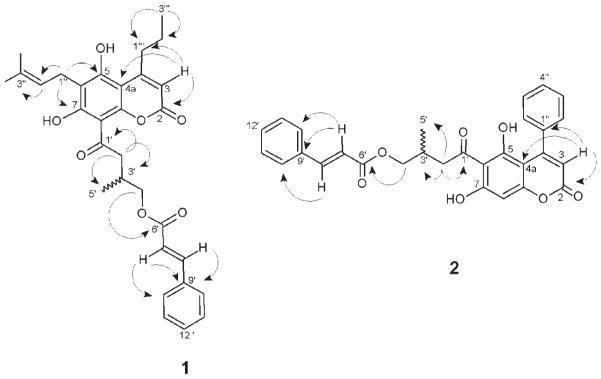
Compound 1 was isolated as a yellowish powder with (c, 0.1, MeOH). The HREIMS of 1 showed a molecular peak ion at m/z 517.2269 [M – H]− supporting a molecular composition of C31H34O7, with 15 degrees of unsaturation. On the basis of the 1 H and 13 C NMR spectra and HMBC correlations of 1 (Table 1, Fig. 2), it was possible to observe a characteristic singlet at δH 5.99 and δC 108.4 (C-3), which correlated to a carbonyl at C-2 (δC 160.8) and to an aromatic carbon at C-4a (δC 103.7), along with two hydroxyl groups at the carbons C-5 and C-7 at δC 161.6 and δC 165.6, respectively, suggesting a 5,7-dihydroxy coumarin skeleton [6, 7]. In addition, the NMR data also showed the presence of an acyl side chain characterized by the presence of 1 H-1 H COSY and HMBC correlations between the methylene group at H-2′ (dd, δH 3.20, J = 15.5, 16.0 Hz and δH 3.38, J = 15.5, 7.4 Hz, respectively), the methine at H-3′ (m, δH 2.66), the methyl group at H-5′ (d, δH 1.13, J = 6.8 Hz), and the methylene at H-4′ (t, δH 4.20, J = 6.1 Hz). The cinnamoyl moiety linked to C-4′ (δC 68.9) was observed by the presence of the protons H-7′ (d, 6.37, J = 16.0 Hz) and H-8′ (d, 7.55, J = 16.0) showing long-range correlations to the aromatic ring signals at C-9′ (δC 134.5) and C-10′ (δC 127.9). The attachment of this acyl side chain at C-8 was proposed due to the small bathochromic shift in the UV spectra after alkali addition, as described for other acylcoumarins [7].
Table 1.
1 H and 13 C NMR data for compounds 1 and 2 (600 and 150 MHz, CD3OD).
| Pos. | 1 δC, type |
δH, m (J in Hz) | HMBC | 2 δC, type |
δH, m (J in Hz) | HMBC |
|---|---|---|---|---|---|---|
| 2 | 160.8, C | 160.0, C | ||||
|
| ||||||
| 3 | 108.4, CH | 5.99, s | 2, 4a, 1‴ | 110.4, CH | 5.91, s | 2, 4a, 1″ |
|
| ||||||
| 4 | 142.6, C | 157.9, C | ||||
|
| ||||||
| 4a | 103.8, C | 102.3, C | ||||
|
| ||||||
| 5 | 161.6, C | 163.7, C | ||||
|
| ||||||
| 6 | 93.0, C | 103.6, C | ||||
|
| ||||||
| 7 | 165.5, C | 167.2, C | ||||
|
| ||||||
| 8 | 112.3, C | 99.3, CH | 6.06, s | 4a | ||
|
| ||||||
| 8a | 156.7, C | 158.2, C | ||||
|
| ||||||
| 1′ | 204.1, C | 204.0, C | ||||
|
| ||||||
| 2′ | 48.4, CH2 | 3.20, dd (15.5, 6.0) 3.38, dd (15.5, 7.4) |
1′,3′,4′,5′ | 48.1, CH2 | 3.23, dd (15.8, 7.3) 3.42, dd (15.8, 6.1) |
1′,3′,5′, 4′ |
|
| ||||||
| 3′ | 30.6, CH | 2.66, m | 2′,4′,5′ | 30.4, CH | 2.68, m | 1′,2′,5′, 4′ |
|
| ||||||
| 4′ | 69.0, CH2 | 4.20, t (6.1) | 2′, 3′, 4′, 6′ | 68.9, CH2 | 4.21, t (6.1) | 2′, 3′, 5′, 6′ |
|
| ||||||
| 5′ | 16.3, CH3 | 1.13, d (6.8) | 2′, 3′, 5′ | 16.3, CH3 | 1.03, d (6.8) | 2′, 3′, 4′ |
|
| ||||||
| 6′ | 167.2, C | 167.2, C | ||||
|
| ||||||
| 7′ | 117.4, CH | 6.37, d (16.0) | 6′, 8′, 9′ | 117.4, CH | 6.43, d (16.0) | 6′, 8′, 9′ |
|
| ||||||
| 8′ | 144.8, CH | 7.55, d (16.0) | 6,7′, 9′,10, 14′ |
144.9, CH | 7.60, d (16.0) | 5′, 7′, 9′, 10′, 14′ |
|
| ||||||
| 9′ | 134.4, C | 134.5, C | ||||
|
| ||||||
| 10′/14′ | 128.0, CH | 7.53 | 8′, 12′ | 128.0, CH | 7.57 | |
|
| ||||||
| 11′/13′ | 128.7, CH | 7.39* | 9′ | 128.7, CH | 7.39* | |
|
| ||||||
| 12′ | 130.1, CH | 7.38* | 130.2, CH | 7.35* | ||
|
| ||||||
| 1″ | 21.0, CH2 | 3.29, d (7.2) | 5, 7, 8, 2″, 12 | 139.9, C | ||
|
| ||||||
| 2″ | 121.7, CH | 5.02, m | 1′, 5′, 3′, 4′ | 127.0, CH | 7.27* | |
|
| ||||||
| 3″ | 131.8, C | 127.2, CH | 7.34* | |||
|
| ||||||
| 4″ | 24.6, CH3 | 1.65, s | 2′, 3′, 5′ | 127.8, CH | 7.34* | |
|
| ||||||
| 5″ | 16.8, CH3 | 1.75, s | 2′, 3′, 4′ | 127.2, CH | 7.34* | |
|
| ||||||
| 6″ | 127.0, CH | 7.27* | ||||
|
| ||||||
| 1″ | 38.9, CH2 | 3.01 t (7.2) | 3, 4a, 5, 2‴, 3‴ | |||
|
| ||||||
| 2″ | 23.2, CH2 | 1.65, m | 1″, 3″ | |||
|
| ||||||
| 3″ | 13.1, CH3 | 1.02, t (7.3) | 1″, 2″ | |||
Overlapped signals
Fig. 2.
Key HMBC correlations for compounds 1 and 2.
The presence of a prenyl group at position 6 was supported by the characteristic doublets at δH 3.29 (J = 7.2 Hz, H-1′′) correlating to the hydroxyl carbons C-5 (δC 161.6) and C-7 (165.6) (Table 1). The signal assigned to H-2′′ (m, δH 5.02), which exhibits longrange correlations to the vinylic carbon at δC 131.8 (C-3′′) and the methyl groups at δC 24.7 (C-4′′) and δC 16.7 (C-5′′), confirmed the prenyl moiety. Besides that, compound 1 showed a propyl group at position 4 of the 5,7-dihydroxy coumarin ring, suggested by the HMBC correlations between the methylene protons at δH 3.01 (t, J = 7.2 Hz, H-1′′′) and the carbons C-3 (δC 108.4) and C-4a (δC 103.7), along with the long-range correlation between the proton at H-3 (s, δH 5.99) to the methylene carbon at C-1′′′ (δC 38.9). The additional signals at δH 1.65 (m, H-2′′′) and δH 1.62 (t, J = 7.3 Hz, H-3′′′) confirmed the structure of the propyl group placed at position 4. All of these assignments led to the structure of 1 as 5,7-dihydroxy-6-(3-methyl-2-butenyl)-8-(4-cinnamoyl-3-methyl-1-oxobutyl)-4-propyl-coumarin, a cinnamoyl ester of 7 (mammein).
Compound 2 was isolated as a yellowish powder with (c, 0.1, MeOH). The HREIMS of 2 showed a molecular ion at m/z 485.1602 [M + H]+ supporting a molecular composition of C29H24O7, with 18 degrees of unsaturation. The 1 H and 13 C NMR spectra (Table 1, Fig. 2) of 2 showed characteristic signals of the 5,7-dihydroxy coumarin skeleton, as described for compound 1. An acyl side chain was characterized by the COSY 1 H-1 H correlations between the proton H-3′ (m, δH 2.68) to the methylene groups H-4′ (qd, δH 4.21, J = 10.8, 6.2 Hz) and H-2′ (dd, δH 3.23, J = 15.8, 7.3 Hz and δH 3.42, J = 15.8, 6.1 Hz, respectively), and an isolated methyl resonating at δH 1.03 (d, J = 6.8 Hz, H-5′). The HMBC correlations of the protons H-3′ and H-2′ to C-1′ (δC 204.1) supported the position of the carbonyl group linked to C-6 (δC 103.6) on the dihydroxy 4-phenyl coumarin skeleton. The O-cinnamoyl group was characterized by the trans-vinyl protons at δH 6.43 (d, J = 16.0 Hz, H-7′) and δH 7.60 (d, J = 16.0 Hz, H-8′), which correlate to the aromatic carbon at δC 134.4 (C-9′) and the ester carbon at δC 167.0 (C-6′). In contrast to compound 1, which showed a prenyl group at position 8, compound 2 exhibited no substituent at this position, which was confirmed by the presence of a singlet at δH 6.06 (H-8) correlated to the aromatic carbon C-4a (δC 102.3). The large bathochromic shift after alkali addition confirmed the position of the side chain at C-6 [7]. On the basis of these assignments, the structure of 2 was established as 5,7-dihydroxy-6-(4-cinnamoyl-3-methyl-1-oxobutyl)-4-phenylcoumarin.
The other known compounds were isolated and their structures were determined by comparing spectroscopic data with literature values. They were identified as mammeigin (3) [8, 9], hydroxymammeigin (4) [9], mammeisin (5) [6], cinnamoyloxymammeisin (6) [7], mammein (7) [10], and the benzophenone ent-nemorosone (8) [11]. All compounds were tested in the NCI 60-cell panel at an initial concentration of 10−5 M. As shown in Table 2, at this concentration, compounds 5 and 7 showed a higher mean percent of inhibition, with 56 and 83 % growth inhibition, respectively, and were submitted to the full five-dose screen; however, their cell line selectivity was modest (see Supporting Information). Nonetheless, a COMPARE [12, 13] study demonstrated a substantial correlation between the cell growth inhibition pattern of the crude geopropolis extract and that of compounds 5 and 7 (Table 3). Some studies have demonstrated that the antiproliferative activity of synthetic coumarins might be attributed to the presence of the hydroxyl group at C-7 [14]. However, there is no report about the influence of phenyl or propyl at C-4 and how those groups would change the activity of these compounds. In the same way, the presence of the cinnamoyl moiety seems to reduce the antiproliferative activity of those coumarins.
Table 2.
Cytotoxicity of compounds in the NCI 60-cell screen.
| Compound | Mean percent inhi- bition at 10−5M |
Percent range at 10−5M |
GI50 against COLO205 (μM) |
GI50 against KM12 (μM) |
|---|---|---|---|---|
| 1 | 0 | 42 | NTa | NT |
|
| ||||
| 2 | 4 | 55 | NT | NT |
|
| ||||
| 3 | 0 | 28 | NT | NT |
|
| ||||
| 4 | 8 | 46 | NT | NT |
|
| ||||
| 5 | 56 | 126 | 9.7 | 12.0 |
|
| ||||
| 6 | 21 | 73 | NT | NT |
|
| ||||
| 7 | 83 | 103 | 10.7 | 10.9 |
|
| ||||
| 8 | 7 | 48 | NT | NT |
|
| ||||
| Adriamycinb | 40.0 ± 1.3b (n = 16) | 105.5 ± 5.6b | 0.098 (n = 2) | 0.162 (n = 2) |
NT: Not tested;
one dose test at 2.5 × 10−7 M
Table 3.
Pearson correlation coefficients at the GI50 level for geopropolis extract and compounds 5 and 7 in the NCI 60-cell screen.
| N192723 extract | 5 | 7 | |
|---|---|---|---|
| N192723 extract | 1 | 0.73 | 0.73 |
|
| |||
| 5 | 0.73 | 1 | 0.85 |
|
| |||
| 7 | 0.73 | 0.85 | 1 |
Both coumarins and benzophenones have been reported to have antiproliferative activity [15–17]. However, the cinnamic acid esters of coumarins have no reported biological activity.
The elucidation of the compounds present in geopropolis hints at the possible botanical origin of the geopropolis. The known coumarins reported herein were previously isolated from plants of the genus Mammea (Clusiaceae) [6] and recently reported as major components of Kielmeyera (Clusiaceae) [7–9]. Ent-nemorosone (8) was recently synthesized; its enantiomer nemorosone is well known from the Clusiaceae [11]. The genus Kielmeyera is native to the state of Bahia, which is the place of collection of our geopropolis samples, which leads us to propose that the bees preferentially visit Clusiaceae plant species in this area to collect the resin with which they make geopropolis. The NCI 60 data also supports this preference, as a COMPARE study using the geopropolis extract data as a seed returned a predominance (10/14) of Clusiaceae extracts out of all plant extracts tested, with Pearson coefficients of > 0.60 (Table 1 S, Supporting Information). Interestingly, the presence of prenylated benzophenones from Clusiaceae was previously reported in A. mellifera propolis type 6 [18], indicating that bees from different species may utilize the same plant sources for collecting propolis. Also, the type of coumarins we found have been reported as insecticidal compounds, indicating that the bees may use coumarin-containing resins to protect the hive from intruding insects [19]. Further, this is the first report describing coumarins as major components of any kind of Brazilian propolis. Last, most of the compounds described here have no previous report of biological activity, so this is the first report on their pharmacological properties.
Materials and Methods
General procedures
Optical rotations ([α]D) were measured on a Perkin-Elmer 241 polarimeter in a 100 × 2 mm cell (units 10−1 deg cm2 g−1). LCMS data were obtained using a Hewlett-Packard Series 1100 MSD, whereas HREIMS data were acquired on an Agilent 6520 Accurate Mass Q-TOF instrument with internal reference masses calibrated at 121.050 87 and 922.009 79, both within 5 ppm. The NMR experiments were performed on a Bruker 600 MHz NMR spectrometer. 1 H and 13 C spectra were referenced to deuterated solvent peaks. The 60 × 2.5 cm i. d. Sephadex LH-20 columns attached to a model UA-6 UV detector and Foxy 200 fraction collector (Teledyne Isco) were used for fractionation of the extract, whereas purification of the compounds was performed using a Varian ProStar 210/215 solvent delivery module HPLC equipped with a Varian ProStar 325 UV-vis detector, operating under Star 6.41 chromatography workstation software. All solvents and chemicals were of analytical grade.
Extraction and isolation
Crude samples of geopropolis from M. scutellaris (native stingless bee) were obtained from the coastal area of the city of Entre Rios (12°22′S and 37°54′W), state of Bahia, Northeast Brazil. Samples of M. scutellaris bee were deposited in the Paulo Nogueira Neto Entomological Collection of the Biosciences Institute at the State University of São Paulo (CEPANN – IBUSP, Brazil) and identified under the voucher number CEPANN 42.863. In addition, this research had authorization and remittance of genetic heritage components granted by the Brazilian National Council of Technological and Scientific Development – CNPq # 010 666/2014–1.
Geopropolis samples were extracted using ethanol 70 % (1 : 7, w/v) and dried as described elsewhere [20]. Two grams of this ethanolic extract of geopropolis (EEGP, NSC# N192723) was coated on diol bonded phase media and eluted with a series of solvents of increasing polarity (hexane, dichloromethane, ethyl acetate, acetone, and methanol) yielding five fractions (A–E) of 40, 500, 170, 60, and 870 mg, respectively. Fraction B was the most active and was selected for further fractionation and isolation of compounds.
Fraction B (89.6 mg) was chromatographed on a 60 × 2.5 cm i. d. Sephadex LH-20 column and eluted with CH2Cl2/MeOH (1 : 1, v/v), with 300 drop fractions collected in each tube. On the basis of TLC and UV traces, they were combined into three fractions (B1, B2, and B3). Fractions B2 and B3 showed activity and were further purified by HPLC. The compounds were isolated using a semipreparative (10 × 250 mm, 5 μm) cyano column with a hexane/isopropanol gradient (0–3 min: 95 % hexane; 3–24 min: 95–80 % hexane, 24–26 min: 80 % hexane, 26–29 min: 80–95 % hexane, 29–31 min: 95 % hexane, flow rate 4 mL/min) as the solvent and the UV detector at λ = 230 nm. One unknown 4-propyl-coumarin, 1 (0.4 mg), one unknown 4-phenyl-coumarin, 2 (1.1 mg), five known coumarins, 3 (1.5 mg), 4 (2.2 mg), 5 (1.5 mg), 6 (11.4 mg), and 7 (0.5 mg), and the benzophenone 8 (0.6 mg) were obtained.
Isolates
5,7-dihydroxy-6-(3-methyl-2-butenyl)-8-(4-cinnamoyl-3-methyl-1-oxobutyl)-4-propylcoumarin
(1; NSC# 781 047): yellow solid; (c, 0.1, MeOH); 1 H and 13 C (600 MHz, CD3OD) NMR data, see Table 1; HREIMS [M – H]− at m/z 517.2269 (calcd. for C31H33O7, 517.2232).
5,7-dihydroxy-6-(4-cinnamoyl-3-methyl-1-oxobutyl)-4-phenylcoumarin
(2; NSC# 781 048): yellow solid; (c, 0.1, MeOH); 1 H and 13 C (600 MHz, CD3OD) NMR data, see Table 1; HREIMS [M + H]+ at m/z 485.1602 (calcd. for C29H25O7, 485.1595). Mammeigin (3; NSC# 781 045): a yellow solid; (c, 0.12, MeOH); the 1 H and 13 C NMR data were identical with those reported elsewhere [8, 9].
Hydroxymammeigin
(4; NSC# 781 050): a yellow solid; (c, 0.23, MeOH); the 1 H and 13 C NMR data were identical with those reported elsewhere [9].
Mammeisin
(5; NSC# 781 046): a yellow solid; (c, 0.63, MeOH); the 1 H and 13 C NMR data were identical with those reported elsewhere [6].
Cinnamoyloxy-mammeisin
(6; NSC#781 051): a yellow solid; the 1 H and 13 C NMR data were identical with those reported else-where [7].
Mammein
(7; NSC# 781 049): a yellow solid; the 1 H and 13 C NMR data were identical with those reported elsewhere [10].
ent-Nemorosone
(8; NSC# 781 044): a white solid; (c, 0.05, CHCl3); the 1 H and 13 C NMR data were identical with those reported recently [11].
Cytotoxicity assay on colon cancer cells
The isolation of the compounds was bioguided by the activity against colon cancer cell lines COLO205 and KM12 in a two-day drug exposure with a formazan (XTT) endpoint, developed by the MTL Assay Development and Screening Section. Cells were cultured in RPMI-1640 medium supplemented with 2 mM L-glutamine and 10 % fetal bovine serum, and held at 37 °C in a humidified incubator with an atmosphere of 5 % CO2 and 95 % air. Cells used in the assay were harvested with RPMI-1640 medium, without phenol red, and supplemented with 2 mM L-glutamine and 10 % fetal bovine serum without antibiotics. After harvest, cells were counted using a Cellometer Auto T4 cell counter, plated in 384-well flat-bottom polystyrene microtiter plates at a density of 5000 cells/well and then were incubated in a 5 % CO2, 95 % air, and 37 °C incubator for 24 h. After incubation, 2-fold serial dilutions of the samples were added to plates using a Biomek FX robotic liquid handling workstation. After a 48-h incubation period, cell viability was accessed with a formazan (XTT reagent) endpoint [13].
NCI 60 data was generated as previously reported [12]. The positive control standard was adriamycin (NSC#123 127). The historic mean GI50 value for the control was 93.5 nM (n = 1816).
Supplementary Material
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and by FAPESP (#2011/23 635–6 and #2012/22 002–2). The authors are grateful to Mr. José Emídio Borges de Souza for providing the geopropolis samples. We thank D. Newman for help in documenting the samples, the Natural Products Support Group at NCI-Frederick, and S. Tarasov, M. Dyba (Biophysics Resource Core, Structural Biophysics Laboratory, CCR), and H. Bokesch (MTL) for assistance with high-resolution mass spectrometry as well as Kirk Gustafson for NMR support. We also thank Dr. Gordon Cragg for making the execution of this work possible.
Footnotes
Supporting information available online at http://www.thieme-connect.de/products
NMR spectra of compounds 1 and 2 and NCI 60 data for all compounds are available as Supporting Information.
Conflict of Interest
The authors have no conflicts of interest to declare.
References
- 1.Sforcin JM, Bankova V. Propolis. Is there a potential for the development of new drugs? J Ethnopharmacol. 2011;133:253–260. doi: 10.1016/j.jep.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 2.Silva BB, Rosalen PL, Cury JA, Ikegaki M, Souza VC, Esteves A, Alencar SM. Chemical composition and botanical origin of red propolis, a new type of Brazilian propolis. Evid Based Complement Alternat Med. 2008;5:313–316. doi: 10.1093/ecam/nem059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dutra RP, Nogueira AMC, Marques RRO, Costa MCP, Ribeiro MNS. Pharmacognostic evaluation of geopropolis of Melipona fasciculata Smith from Baixada maranhense, Brazil. Rev Bras Farmacogn. 2008;18:557–562. [Google Scholar]
- 4.Franchin M, da Cunha MG, Denny C, Napimoga MH, Cunha TM, Bueno-Silva B, Alencar SM, Ikegaki M, Rosalen PL. Bioactive fraction of geopropolis from Melipona scutellaris decreases neutrophils migration in the inflammatory process: involvement of nitric oxide pathway. Evid Based Complement Alternat Med. 2013;2013:907041. doi: 10.1155/2013/907041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.da Cunha MG, Franchin M, de Carvalho Galvão LC, de Ruiz AL, de Carvalho JE, Ikegaki M, Alencar SM, Koo H, Rosalen PL. Antimicrobial and antiproliferative activities of stingless bee Melipona scutellaris geopropolis. BMC Complement Altern Med. 2013;28:13–23. doi: 10.1186/1472-6882-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crombie L, Games DE, McCormick A. Extractives of Mammea americana L. Part II. The 4-phenylcoumarins. Isolation and structure of mammea A/AA, A/A cyclo D A/BA A/AB, and A/BB. J Chem Soc C. 1967:2553–2559. [Google Scholar]
- 7.Cruz FG, Moreira LM, David JM, Guedes MLS, Chávez JP. Coumarins from Kielmeyera reticulata. Phytochemistry. 1998;47:1363–1366. [Google Scholar]
- 8.Cruz FG, Silva-Neto JT, Guedes MLS. Xanthones and coumarins from Kielmeyera lathrophyton. J Braz Chem Soc. 2001;12:117–122. [Google Scholar]
- 9.Gramacho RS, Nagem TJ, Oliveira TT, Queiroz MELR, Neves AA, Saddi N. Phenylcoumarins from Kielmeyera elata. Phytochemistry. 1999;51:579–581. [Google Scholar]
- 10.Djerassi C, Eisenbraun EJ, Finnegan RA, Gilbert B. Naturally occurring oxygen heterocyclics. VII. The structure of mammein. J Org Chem. 1960;25:2164–2169. [Google Scholar]
- 11.Sparling BA, Tucker JK, Moebius DC, Shair MD. Total synthesis of (−)-nemorosone and (+)-secohyperforin. Org Lett. 2015;17:3398–3401. doi: 10.1021/acs.orglett.5b01121. [DOI] [PubMed] [Google Scholar]
- 12.Paull KD, Shoemaker RH, Hodes L, Monks A, Scudiero DA, Rubinstein L, Plowman J, Boyd MR. Display and analysis of patterns of differential activity of drugs against human tumor cell lines: development of mean graph and COMPARE algorithm. J Natl Cancer Inst. 1989;81:1088–1092. doi: 10.1093/jnci/81.14.1088. [DOI] [PubMed] [Google Scholar]
- 13.Shoemaker RH. The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer. 2006;6:813–823. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Wang X, Xu G, Zeng L, Cheng K, Gao P, Sun Q, Liao W, Zhang J. Synthesis and biological evaluation of a novel class of coumarin derivatives. Bioorg Med Chem Lett. 2014;24:5274–5278. doi: 10.1016/j.bmcl.2014.09.051. [DOI] [PubMed] [Google Scholar]
- 15.Finnegan RA, Merkel KE, Back N. Constituents of Mammea americana L. 8. Novel structural variations on the mammein theme and antitumor activity of mammein and related coumarin and phloroglucinol derivatives. J Pharm Sci. 1972;61:1599–1603. doi: 10.1002/jps.2600611011. [DOI] [PubMed] [Google Scholar]
- 16.López-Pérez JL, Olmedo DA, Del Olmo E, Vásquez Y, Solís PN, Gupta MP, A San Feliciano. Cytotoxic 4-phenylcoumarins from the leaves of Marila pluricostata. J Nat Prod. 2005;68:369–373. doi: 10.1021/np049642g. [DOI] [PubMed] [Google Scholar]
- 17.Popolo A, Piccinelli AL, Morello S, Sorrentino R, Osmany CR, Rastrelli L, Pinto A. Cytotoxic activity of nemorosone in human MCF-7 breast cancer cells. Can J Physiol Pharmacol. 2011;89:50–57. doi: 10.1139/y10-100. [DOI] [PubMed] [Google Scholar]
- 18.Castro ML, do Nascimento AM, Ikegaki M, Costa-Neto CM, Alencar SM, Rosalen PL. Identification of a bioactive compound isolated from Brazilian propolis type 6. Bioorg Med Chem. 2009;17:5332–5335. doi: 10.1016/j.bmc.2009.04.066. [DOI] [PubMed] [Google Scholar]
- 19.Crombie L, Games DE, Haskins NJ, Reed GF. Extractives of Mammea americana L. V. The insecticidal compounds. J Chem Soc Perkin 1. 1972;18:2255–2260. doi: 10.1039/p19720002255. [DOI] [PubMed] [Google Scholar]
- 20.Franchin M, da Cunha MG, Denny C, Napimoga MH, Cunha TM, Koo H, de Alencar SM, Ikegaki M, Rosalen PL. Geopropolis from Melipona scutellaris decreases the mechanical inflammatory hypernociception by inhibiting the production of IL-1β and TNF-α. J Ethnopharmacol. 2012;143:709–715. doi: 10.1016/j.jep.2012.07.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.