Abstract
We report here a human case of Taenia asiatica infection which was confirmed by genetic analyses in Dali, China. A patient was found to have symptoms of taeniasis with discharge of tapeworm proglottids. By sequencing of the mitochondrial cytochrome c oxidase subunit 1 (cox1) gene, we observed nucleotide sequence identity of 99% with T. asiatica and 96% with T. saginata. Using the cytochrome b (cytb) gene, 99% identity with T. asiatica and 96% identity with T. saginata were found. Our findings suggest that taeniasis of people in Dali, China may be mainly caused by T. asiatica.
Keywords: Taenia asiatica, case report, cox1 gene, cytb gene
INTRODUCTION
Human taeniasis is caused by 3 species of taeniid tapeworms, Taenia solium, Taenia saginata, and Taenia asiatica. Among these 3 species, T. asiatica was recorded as a distinct one from T. saginata in 1993 both of which are morphologically similar to each other [1-3]. T. asiatica is more restrictedly distributed in Asian countries, i.e., Korea, China, Taiwan, Thailand, Indonesia, Vietnam, Japan, and the Philippines [1].
Because of the morphological similarity, T. asiatica had previously been considered as T. saginata [2,3]. Thus, molecular methods became an efficient tool which can provide a clearer phylogenetic resolution. The methods of PCR-RFLP, singlestrand conformation polymorphism (SSCP), a loop-mediated isothermal amplification (LAMP), multiplex PCR, and coproDNA test have been reported [4-9]. These methods have their own advantages accordingly. Their mitochondrial cytochrome c oxidase 1 (cox1) and cytochrome b (cytb) gene sequences have been determined completely, and they have been widely used to study the population structure and genetic differentiation of several tapeworm species [10-12].
A previous study indicated that short sequences of the cox1 gene can give a bias to analysis, and rather a complete sequence data would provide more reliable results [13,14]. Phylogenetic trees can show the relationships among different taxa. In the present study, we report a human case of T. asiatica infection by genetic analyses based on cox1 and cytb genes in Dali, China.
CASE RECORD
The patient (Bai nationality, Dali, China) is a healthy 30-year-old man who visited Dali People’s Hospital after finding a whitish yellow tapeworm segment in his feces on 26 May 2014. He had not experienced any abdominal discomfort or pain, and had not visited any foreign countries recently. He had a history of eating raw pork and raw pig liver mixed with sour sauce and salted garlic. His blood test results were normal.
The man was treated with traditional Chinese medicine (the combination of pumpkin seeds and areca nut extract) and 30% hydrated magnesium sulfate (MgSO4·7H2O) solution. An almost complete cestode (about 1.8 m long) without a scolex was expelled after about 3-6 hr of treatment. This tapeworm specimen was kept in a 50 ml centrifuge tube filled with 0.9% normal saline and forwarded to National Key Laboratory of Veterinary Etiological Biology (Lanzhou, China) for examinations.
Genomic DNA (gDNA) was extracted from the proglottid of the tapeworm using an AxyPrep small genomic DNA kit (Axygen, Beijing, China). Purified gDNA was used as a template for PCR for cox1. The PCR primers were up primer (5´-ATGAGTGTTAAATTTTTGTTAAGTT-3´) and down primer (5´-TTAAACTAAAAAACCAC GAGC-3´). PCR was performed in a 50 µl reaction mixture containing 25 µl of PrimeSTAR® HS Premix (Takara, Kyoto, Japan), 1.5 µl of 10 μM of each primers, 20 µl of distilled water, and 2 µl purified gDNA from tapeworm. The PCR reaction was carried out for 35 cycles of 94˚C for 40 sec, 55˚C for 40 sec, 72˚C for 60 sec. The reaction initial denaturing step at 95˚C for 5 min and terminated with a final extension step at 72˚C for 10 min. The PCR amplification of cytb sequence was performed using a primer pair of cytb: up primer (5´-ATGATTAGATTAT TTCGACG-3´) and down primer (5´-TTAATAAATCTTAAAAAGAAACATAAGC-3´). PCR conditions for cytb gene were the same as those for cox1.
The amplification products were fractionated using 1% (w/v) agarose gel, and then purified (Axygen). The purified products were then ligated into PMD-18T vector (Takara), followed by transformation into Escherichia coli DH5a and sequencing. The sequences for cox1 and cytb were aligned with the corresponding sequences of tapeworms obtained from GenBank database. Molecular identification of the tapeworm specimens was based on a comparison with the nucleotide sequences of cox1 and cytb genes of T. solium, T. Saginata, and T. asiatica. Phylogenetic analyses were performed using the neighbor-joining (NJ) method by MEGA (Version 5) with the Kimura-2 parameter model [15]. Bootstrap analysis was performed with 1,000 replications.
The whitish yellow tapeworm from the patient was shown in Fig. 1. Approximately, 1,620 bp nucleotide sequence from the mitochondrial cox1 gene of the tapeworm in the present study was obtained, which showed an identity of 99% (1 different base) with T. asiatica (AB533175.1) and 96% (65 different bases) with T. saginata (AB465239.1), respectively. The phylogenetic analysis revealed that our tapeworm was closely related to T. asiatica (Fig. 2). Furthermore, approximately 1,068 bp sequence of the mitochondrial cytb gene had an identity of 99% (2 bp differences) with T. asiatica (no. AB066580.1) and 96% (43 bp different bases) with T. saginata, respectively. Phylogenetic analysis showed that the cestode was closely related to T. asiatica (Fig. 3). Sequences of all Taenia specimens derived from different Asian countries formed a monophyletic group.
Fig. 1.

An almost complete strobila (about 1.8 m in lenth), without the scolex, of Taenia asiatica recovered from our patient.
Fig. 2.
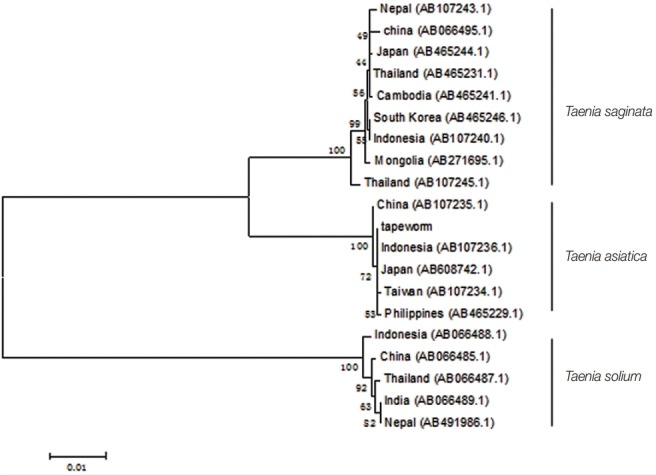
Phylogenetic tree of 3 Taenia spp. using the mitochondrial cox1 gene sequences. Our tapeworm specimen showed 99% identity (1 different base) with T. asiatica (AB533175.1) and 96% identity (65 different bases) with T. saginata (AB465239.1).
Fig. 3.
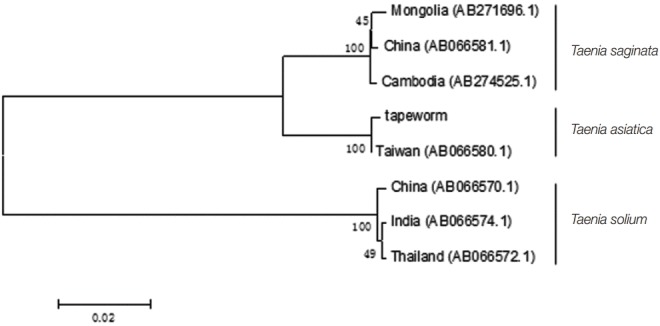
Phylogenetic tree of 3 Taenia spp. using the mitochondrial cytb gene sequences. Our tapeworm specimen showed 99% identity (2 bp differences) with T. asiatica (no. AB066580.1) and 96% identity (43 bp different bases) with T. saginata.
DISCUSSION
To date, taeniasis still occurs in the majority of regions in Southwest China including Yunnan, Sichuan, Guizhou, Qinghai, and many other provinces where a minority of people like to eat raw pork, undercooked beef, and raw pig liver mixed with sour sauce and salted garlic, and the prevalence and incidence of human taeniasis remains unknown in southwest China [1,16-18]. Eating raw liver which contains cysticerci is the main risk factor of tapeworm infection. In the present study, since infection of the patient was a result of consumption of undercooked pig liver, it is reasonable to guess that the taeniasis is due to T. asiatica caused in Dali of Yunnan Province. Neither T. saginata nor T. solium were detected during the same period, so T. asiatica was considered to be the dominant species causing human taeniasis in Dali.
As the living standards of people improve, the incidence of taeniasis became markedly lower in Dali, but the local Bai people still have the habit of eating raw pork and raw pig liver. So, it is necessary for them to know the dangers of taeniasis and to understand the routes of tapeworm transmission through continuous public health educations. There are 2 strategies for cutting off the domestic life of T. asiatica. First, local people should pay attention to prevent human taeniasis avoiding intake of uncooked or undercooked pig liver. Second, the patient who is infected with the tapeworm must be treated early with anthelmintics and keep pigs without contacting human feces. We believe that the taeniasis will be eliminated someday in the future if native people take above these 2 advices in Dali.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 31372433), the Science Fund for Creative Research Groups of Gansu Province, China (no. 1210RJIA006) and the opening projects of National Key Laboratory of Veterinary Etiological Biology at Lanzhou Veterinary Research Institute of Chinese Academy of Agricultural Sciences, China (grant no. 201001).
Footnotes
We have no conflict of interest related to this study.
REFERENCES
- 1.Eom KS, Jeon HK, Rim HJ. Geographical distribution of Taenia asiatica and related species. Korean J Parasitol. 2009;47:115–124. doi: 10.3347/kjp.2009.47.S.S115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galan-Puchades MT, Fuentes MV. Diagnosis of human cysticercosis and Taenia asiatica. Am J Trop Med Hyg. 2009;81:1165. doi: 10.4269/ajtmh.2009.09-0398a. [DOI] [PubMed] [Google Scholar]
- 3.Eom KS, Rim HJ. Morphologic descriptions of Taenia asiatica sp. n. Korean J Parasitol. 1993;31:1–6. doi: 10.3347/kjp.1993.31.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Bowles J, McManus DP. Genetic characterization of the Asian Taenia, a newly described taeniid cestode of humans. Am J Trop Med Hyg. 1994;50:33–44. [PubMed] [Google Scholar]
- 5.Gass RB, Chilton NB. Characterisation of taeniid cestode species by PCR-RFLP of ITS2 ribosomal DNA. Acta Trop. 1995;59:31–40. doi: 10.1016/0001-706x(94)00085-f. [DOI] [PubMed] [Google Scholar]
- 6.Gass RB, Zhu X, Woods W. Genotyping Taenia tapeworms by single-strand conformation polymorphism of mitochondrial DNA. Electrophoresis. 1999;20:2834–2847. doi: 10.1002/(SICI)1522-2683(19991001)20:14<2834::AID-ELPS2834>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez LM, Montero E, Harrison LJ, Parkhouse RM, Garate T. Differential diagnosis of Taenia saginata and Taenia solium infection by PCR. J Clin Microbiol. 2000;38:737–744. doi: 10.1128/jcm.38.2.737-744.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nkouawa A, Sako Y, Nakao M, Nakaya K, Ito A. Loop-mediated isothermal amplification method for differentiation and rapid detection of Taenia species. J Clin Microbiol. 2009;47:168–174. doi: 10.1128/JCM.01573-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamasaki H, Allan JC, Sato MO, Nakao M, Sako Y, Nakaya K, Qiu D, Mamuti W, Craig PS, Ito A. DNA differential diagnosis of taeniasis and cysticercosis by multiplex PCR. J Clin Microbiol. 2004;42:548–553. doi: 10.1128/JCM.42.2.548-553.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gasser RB, Zhu X, Woods W. Genotyping Taenia tapeworms by single-strand conformation polymorphism of mitochondrial DNA. Electrophoresis. 1999;20:2834–2837. doi: 10.1002/(SICI)1522-2683(19991001)20:14<2834::AID-ELPS2834>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 11.Nakao M, Okamoto M, Sako Y, Yamasaki H, Nakaya K, Ito A. A phylogenetic hypothesis for the distribution of two genotypes of the pig tapeworm Taenia solium worldwide. Parasitology. 2002;124:657–662. doi: 10.1017/s0031182002001725. [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Hernandez F, Jimenez-Gonzalez DE, Chenillo P, Alonso-Femandez C, Maravilla P, Flisser A. Geographical widespread of two lineages of Taenia solium due to human migrations: can population genetic analysis strengthen this hypothesis? Infect Genet Evol. 2009;9:1108–1114. doi: 10.1016/j.meegid.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Jeon HK, Lee KH, Kim KH, Hwang UW, Eom KS. Complete sequence and structure of the mitochondrial genome of the human tapeworm, Taenia asiatica (Platyhelminthes; Cestoda) Parasitology. 2005;130:717–726. doi: 10.1017/s0031182004007164. [DOI] [PubMed] [Google Scholar]
- 14.Jeon HK, Eom KS. Taenia asiatica and Taenia saginata: genetic divergence estimated from their mitochondrial genomes. Exp Parasitol. 2006;113:58–61. doi: 10.1016/j.exppara.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 15.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Tao H, Zhang B, Wang H, Wang Y, Li Z, Yang J, Yang B, Li Y, Pang Y, Zhang H, Wu Y. First discovery of Taenia saginata asiatica infection in Yunnan province. Chin J Parasitol Parasit Dis. 1999;17:95–96. (in Chinese) [PubMed] [Google Scholar]
- 17.Wang ZR, Bao HE. Identification of Taenia saginata by mtCO I in four areas of Yunnan and Guizhou provinces. Chin J Parasitol Parasit Dis. 2003;21:20–23. (in Chinese) [PubMed] [Google Scholar]
- 18.Li T, Craig PS, Ito A, Chen X, Qiu D, Qiu J, Sato MO, Wandra T, Bradshaw H, Li L, Yang Y, Wang Q. Taeniasis/cysticercosis in a Tibetan population in Sichuan Province, China. Acta Trop. 2006;100:223–231. doi: 10.1016/j.actatropica.2006.11.003. [DOI] [PubMed] [Google Scholar]


