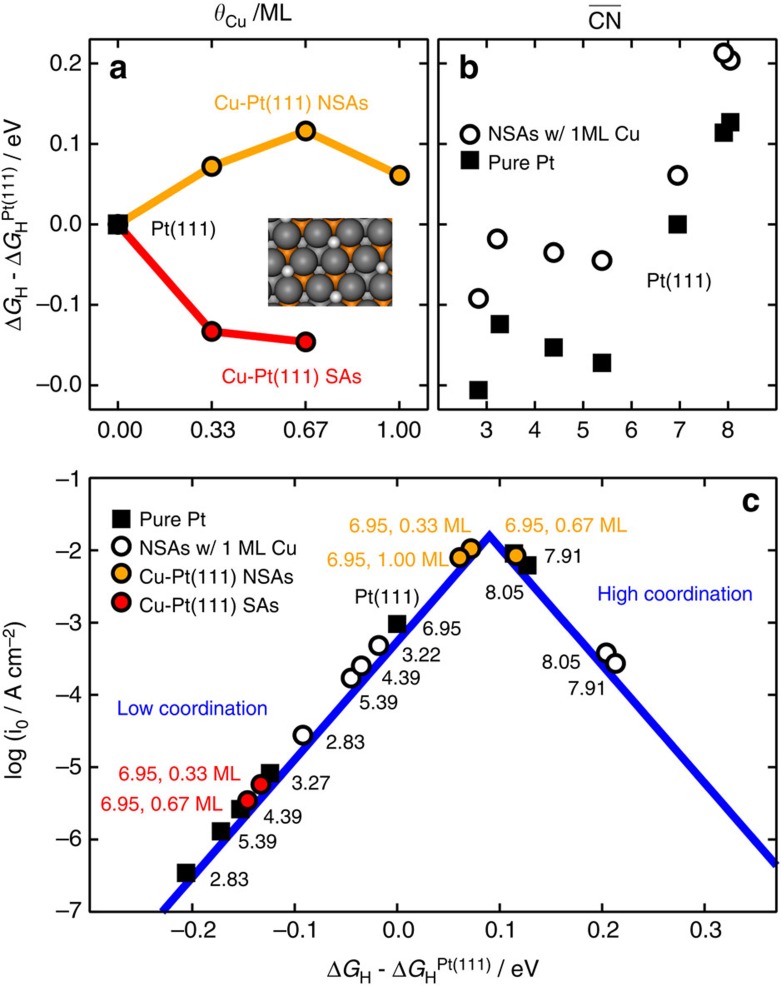
Figure 7. Trends in H2 adsorption and evolution.
(a) *H adsorption energies with surface Cu (SAs, red) and subsurface Cu (NSAs, orange) on Pt(111). *H binds more strongly to SAs compared with Pt(111), whereas the opposite is observed for the NSAs. The inset shows *H at a NSA with 0.67 ML Cu (grey balls, Pt; red, Cu atoms; white, adsorbed *H). (b) Adsorption energies of *H on Pt and NSAs with 1ML Cu as a function of the generalized coordination numbers of the active sites. In all cases, *H adsorption energies are weaker on the NSAs than on the corresponding sites on Pt. Moreover, more coordinated sites than in the case of (111) terraces bind *H weaker, whereas the less coordinated ones bind it stronger. (c) Volcano plot showing the individual HER activity, coordination and composition of all studied sites. Sites at Cu-Pt(111) NSAs and overcoordinated Pt sites possess the highest activities, whereas SAs and undercoordinated sites are not active.