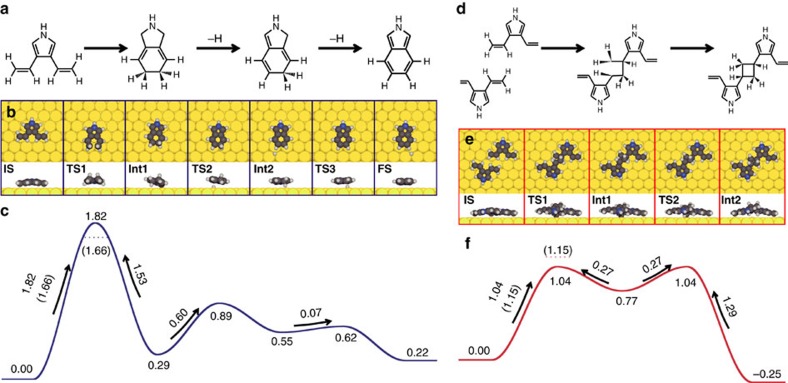
Figure 6. Reaction pathway considerations for a model pyrrole molecule on Au(111).
(a–c) Monomer cyclization. (d–f) Initial steps of the dimerization process. For both reactions, it is assumed that the ethyl legs have been transformed into ethenyl groups through dehydrogenation reactions, the details of which are shown in the Supplementary Information. (a,d) Chemical stick models and (b,e) top and side view ball models for reactants (IS), transition states (TS), reaction intermediates (Int) and the monomer reaction final state (FS). (c,f) Energy profiles for the monomer cyclization and initial steps of the dimerization process, respectively. Free energy barriers are shown in parentheses and indicated by the dotted lines for TS1 of each reaction, calculated by including vibrational enthalpy and entropy at 275 °C. Energies are given in units of eV.