Table 1. X-Ray Data Collection, Phasing and Refinement Statistics.
| Data collection | |
| Wavelength (Å) | 0.9791 |
| Space group | C2 |
| Unit cell a, b, c (Å) | 106.04, 98.10, 154.72 |
| β = 96.04° | |
| Resolution (Å) | 30.0–2.70 (2.80–2.70) |
| Total observations | 326520 (31587) |
| Unique reflections | 43536 (4327) |
| Redundancy | 7.5 (7.3) |
| Rmerge (%)* | 9.7 (59.1) |
| I/σ (I) | 14.8 (2.9) |
| Phasing | |
| No. of Se sites | 13 |
| Figure of merit | 0.43 |
| No. of autobuilt residues | 1231 (76%) |
| Refinement | |
| Resolution (Å) | 30.0–2.70 (2.76–2.70) |
| No. of reflections (Working/Free) | 41334/3091 |
| Rwork/Rfree (%)† | 18.9/22.5 (29.3/33.0) |
| R.m.s.d. bond lengths (Å) | 0.010 |
| R.m.s.d. bond angles (°) | 1.6 |
| Ramachandran plot (%)‡ | |
| Favored | 95.5 |
| Allowed | 3.4 |
| Outlier | 1.1 |
| Average B-values (Å2)/ No. of atoms | |
| Protein | 77.7/12071 |
| UDP | 57.1/100 |
| CMP-Neu5Ac | 59.3/164 |
| ManNAc | 94.7/15 |
| Water | 67.3/331 |
Values in parentheses are for the highest resolution shells.
*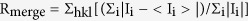 for all equivalent reflections.
for all equivalent reflections.
†Rwork and Rfree were calculated as 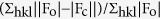 for 95% data used in the refinement and 5% data that were excluded.
for 95% data used in the refinement and 5% data that were excluded.
‡Checked by using MolProbity34.
